What is E466 (CMC/Cellulose Gum)?
Complete guide to this synthetic emulsifier with emerging evidence of gut damage, microbiota disruption, and potential cancer links
The Quick Answer
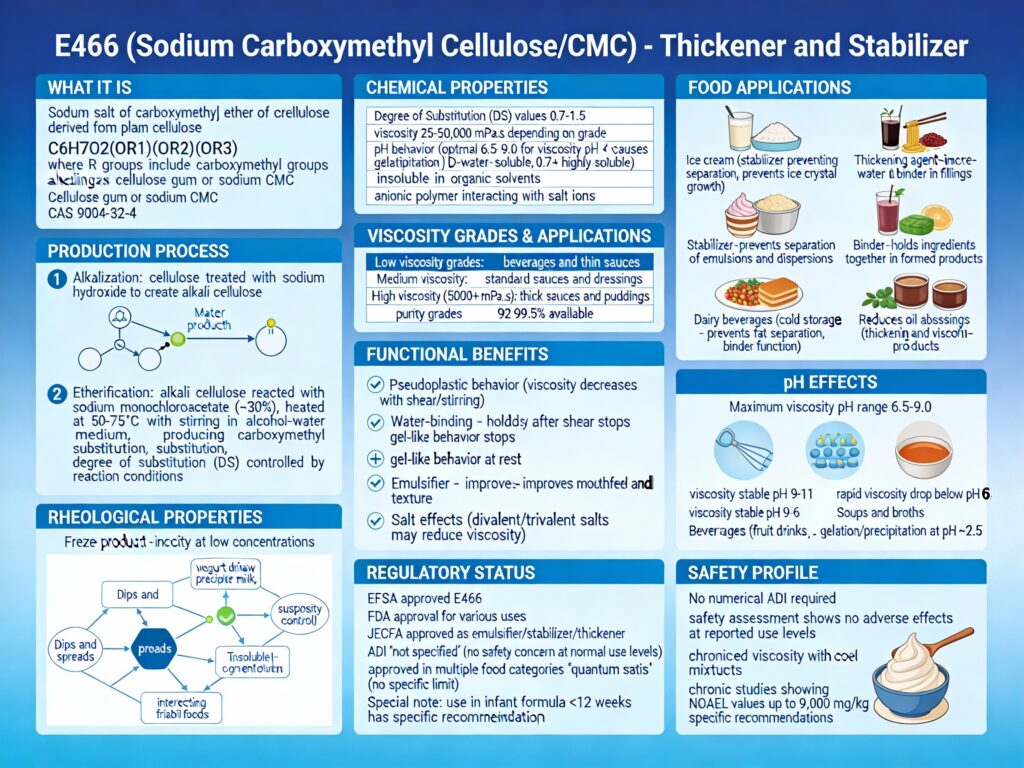
E466 is sodium carboxymethyl cellulose (CMC), also called cellulose gum—a synthetic polymer created by chemically modifying natural cellulose (from wood pulp or cotton fibers) with sodium hydroxide and chloroacetic acid. It’s used as a thickener, emulsifier, and stabilizer in thousands of processed foods.
While regulatory authorities approved CMC as safe, significant emerging research (2015-2024) identifies serious health concerns that regulatory approval predates and doesn’t adequately address. Recent studies show that CMC damages the gut barrier, disrupts the microbiota, promotes inflammatory bowel disease, obesity, metabolic syndrome, and may increase cancer risk—concerns severe enough that independent food safety evaluators classify it as ORANGE—SOME CONCERNS.
Unlike truly safe additives like citric acid or pectin, CMC is a synthetic chemical modification of cellulose that demonstrates clear biological harm in both animal and human studies, making it one of the additives most recommended for avoidance by those prioritizing gut health.
📌 Quick Facts
- Category: Emulsifier, thickener, stabilizer, synthetic food additive
- Composition: Synthetic polymer; cellulose modified with sodium hydroxide and chloroacetic acid
- Found in: Ice cream, yogurt, low-fat foods, sauces, dressings, baked goods, sugar-free products
- Safety Status: FDA GRAS approved; EFSA approved (2017); BUT emerging concerns identified
- Controversy Level: ORANGE—SOME CONCERNS (independent rating); moderate-high emerging health risk
- Emerging Concerns: Gut barrier damage, microbiota disruption, IBD, obesity, metabolic syndrome, cancer links
- Key Mechanism: Acts like a detergent, disrupting the protective mucus layer in the gut
- Critical Timeline: Long approved, but significant health evidence emerged 2015-2024 AFTER regulatory approval
What Exactly Is Carboxymethyl Cellulose (CMC)?
Sodium carboxymethyl cellulose (CMC) is a synthetic polymer created by chemically modifying natural cellulose with sodium hydroxide and chloroacetic acid. The result is a water-soluble powder that binds water and creates thick, stable gels.
Chemical composition: Cellulose is a linear glucose polymer (C₆H₁₀O₅)ₙ. CMC is created by reacting cellulose with sodium hydroxide and chloroacetic acid, which adds carboxymethyl (–CH₂COOH) groups to the cellulose backbone, making it water-soluble. The molecular weight ranges from 17,000 to several hundred thousand Daltons.
In simple terms: It’s wood pulp or cotton fiber chemically modified in a lab to create a synthetic thickening agent. Unlike plant gums that are naturally extracted, CMC is a synthetic chemical modification.
Key properties:
• Synthetic, not natural: Created through chemical modification; not found naturally in foods
• Water-binding: Absorbs and holds water, creating thick gels
• Detergent-like properties: Research suggests it acts like a mild detergent, disrupting the protective mucus barrier in the gut
• Not absorbed: Passes through the digestive system largely intact
• Emulsifying: Helps stabilize oil-water mixtures in foods
• Degree of substitution matters: The number of carboxymethyl groups attached affects its properties; food-grade has 0.2-1.5 groups per glucose unit
⚠️ Critical Timeline: Approval Preceded Health Evidence
This is essential to understanding E466’s safety controversy:
• 1960s-2010s: CMC considered safe; regulatory approval based on older toxicity studies
• 2015: NIH-funded study shows CMC promotes colitis in mice; disrupts gut microbiota
• 2017: EFSA re-evaluated and maintained approval (but assessment may not fully incorporate emerging 2015-2016 microbiota data)
• 2021: Randomized controlled trial in humans shows even 15g daily for 11 days causes gut barrier damage
• 2024: Massive 7-year study of 92,000 French adults links CMC to IBD and cancer risk
• Result: Regulatory approval predates health evidence; agencies slow to reassess in light of new data
Where You’ll Find E466
CMC appears in thousands of processed foods, particularly those requiring smooth texture or water binding:
• Ice cream and frozen desserts
• Yogurt and cultured dairy products
• Low-fat and diet foods (marketed as “healthy”)
• Sugar-free products and sugar-free maple syrup
• Salad dressings and vinaigrettes
• Sauces and gravies
• Mayonnaise and creamy condiments
• Baked goods and bread
• Canned soups and broths
• Cheese and processed cheese
• Fruit fillings and preserves
• Low-fat meat products
• Beverages (some low-calorie drinks)
• Toothpastes and pharmaceuticals
How Is CMC Produced?
CMC production is a chemical synthesis process involving synthetic modification:
Step 1: Starting Material
Natural cellulose is obtained from wood pulp or cotton fibers. While the source is natural, the final product is synthetic.
Step 2: Alkali Treatment
Cellulose is treated with sodium hydroxide (NaOH), which swells the cellulose fibers and activates them for further chemical modification.
Step 3: Carboxymethylation
Chloroacetic acid (ClCH₂COOH) is added to the activated cellulose. This reacts with hydroxyl groups on the cellulose chain, attaching carboxymethyl groups (–CH₂COOH):
Cellulose-OH + NaOH + ClCH₂COOH → Cellulose-O-CH₂COONa + NaCl + H₂O
Step 4: Neutralization
The product is neutralized and washed to remove unreacted chemicals and salts.
Step 5: Precipitation & Separation
The CMC is precipitated and separated from the aqueous solution.
Step 6: Purification & Drying
The product is purified and dried to create the food-grade powder.
Note: This is a multi-step chemical synthesis involving harsh chemicals (sodium hydroxide, chloroacetic acid). The final product is synthetic and structurally different from natural cellulose.
Functions of E466 in Food
CMC serves multiple functions in food manufacturing:
As a thickener: Creates viscosity and smooth texture in sauces, dressings, and liquid foods. Effective at very low concentrations.
As an emulsifier and stabilizer: Prevents separation of oil and water in dressings, mayonnaise, and sauces. Creates uniform, stable emulsions.
As a water-retention agent: Prevents moisture loss in baked goods, yogurt, and ice cream; prevents syneresis (liquid separation).
As a freeze-thaw stabilizer: Controls ice crystal formation in frozen desserts.
Why manufacturers use it: Extremely cost-effective; small amounts create major texture/stability improvements; allows reduction of fat while maintaining mouthfeel; extends shelf life; makes low-fat products taste and feel more luxurious.
Is E466 Safe? Regulatory Approval vs Emerging Research
This is where the CMC story becomes critical: E466 is approved, but mounting evidence (2015-2024) shows serious health risks that regulatory approval predates.
Regulatory Position (Approval-Based):
• FDA: GRAS (Generally Recognized As Safe); approved for use
• EFSA: Approved in 2017; concluded “no safety concern at reported uses and use levels”; no numerical ADI set
• Position: Safe at approved food use levels
Emerging Research Evidence (2015-2024):
Critical 2021 Randomized Controlled Human Trial
A randomized controlled trial published in 2021 tested even modest doses: just 15 grams of CMC per day for only 11 days in healthy volunteers caused:
• Gut barrier damage: Increased intestinal permeability; weakened tight junctions allowing bacteria passage
• Reduced microbiota diversity: Decreased beneficial bacterial species diversity
• Altered microbiota composition: Changed which bacteria predominate
• Increased inflammation markers: Elevated markers of intestinal inflammation
• Abdominal discomfort: Bloating, gas, discomfort in some subjects
Massive 2024 French Cohort Study (92,000 Adults, 7 Years)
A nearly 7-year prospective cohort study of 92,000 healthy French adults published in February 2024 found that CMC exposure was associated with:
• Inflammatory bowel disease: Increased incidence of IBD symptoms and conditions
• Gut microbiota deterioration: Reduced beneficial bacteria; impaired diversity
• Cancer risk: Statistically significant associations with breast, prostate, and colon cancer
• Metabolic dysfunction: Associated with metabolic syndrome markers
Animal Study Findings (Mechanism Understanding)
Four separate animal studies demonstrated:
• Chronic intestinal inflammation: CMC promotes persistent inflammation in the colon
• Dysbiosis: Disrupts healthy gut bacteria composition; promotes harmful species
• Barrier dysfunction: Damages the protective mucus layer lining the intestine
• Cancer promotion: Increases incidence of colon, breast, and prostate cancers in rodents
• Metabolic syndrome: Promotes obesity, insulin resistance, lipid abnormalities
Mechanism: How CMC Damages Gut Health
Research suggests CMC works like a detergent:
• Mucus layer disruption: CMC’s surfactant-like properties thin the protective mucus barrier lining the intestinal wall
• Epithelial exposure: Once mucus is compromised, intestinal bacteria can directly contact epithelial cells
• Immune activation: Bacterial contact triggers inflammatory response and immune activation
• Dysbiosis: The inflammatory environment selects for pathogenic bacteria over beneficial species
• Barrier leakage: Inflammation damages tight junctions between epithelial cells, causing leaky gut
• Cascade effect: Increased bacterial translocation triggers systemic inflammation and metabolic dysfunction
⚠️ Regulatory Inadequacy Identified
In December 2022, EFSA reviewed new data on E466 and found:
“For scenarios (i) and (iii), the exposure to the toxic elements from consumption of E 466 is substantial and this gives rise to concern.”
However, the EFSA’s assessment focused primarily on toxic element contaminants (arsenic, lead, cadmium, mercury) rather than the biological effects of CMC itself on gut health. The agency did NOT comprehensively reassess the gut microbiota and IBD evidence from 2015-2024.
Health Effects & Side Effects
Acute effects (from food consumption):
• Bloating and gas (even at modest doses)
• Abdominal discomfort and cramping
• Diarrhea or constipation (dose-dependent)
• Nausea
Chronic effects (from regular consumption):
• Dysbiosis (altered gut bacteria composition)
• Reduced beneficial bacteria diversity
• Intestinal barrier dysfunction (“leaky gut”)
• Chronic intestinal inflammation
• Inflammatory bowel disease symptoms and increased IBD risk
• Metabolic syndrome (obesity, insulin resistance, dyslipidemia)
• Increased cancer risk (colon, breast, prostate—from studies)
• Impaired immune function
• Reduced vitamin production (gut bacteria produce B vitamins, vitamin K)
High-risk populations:
• Those with existing IBD (Crohn’s disease, ulcerative colitis)
• People with IBS or sensitive digestive systems
• Individuals with metabolic syndrome or obesity
• Those consuming high amounts of processed/low-fat foods
• People with compromised immune function
• Those on antibiotics (already dysbiotic)
CMC vs Related Emulsifiers: Comparative Risk
| Additive | Type | Gut Health Impact | Cancer Risk | Status |
|---|---|---|---|---|
| E466 CMC | Synthetic cellulose ether | ✗ Damages barrier; dysbiosis | ~ Linked in studies | APPROVED but ORANGE—SOME CONCERNS |
| E407 Carrageenan | Seaweed extract | ✗ Damages barrier; pro-inflammatory | ~ Potential links | APPROVED but HIGH CONCERNS |
| E433 Polysorbate 80 | Synthetic surfactant | ✗ Disrupts microbiota; inflammatory | ~ Emerging concerns | APPROVED but CONCERNS |
| E410 Locust Bean Gum | Plant extract | ✓ Prebiotic; supports health | ✓ No concerns | SAFE |
| E440 Pectin | Plant extract | ✓ Prebiotic; protective | ✓ No concerns | SAFE |
The Bottom Line
E466 (sodium carboxymethyl cellulose/CMC) is approved by regulatory authorities but faces serious emerging evidence of gut damage, dysbiosis, IBD, metabolic dysfunction, and potential cancer links that postdate its approval and aren’t fully reflected in regulatory assessment. It’s classified as ORANGE—SOME CONCERNS, and the body of evidence warrants significant caution.
Key takeaways:
• Regulatory approval but emerging health evidence: Approved 2017; serious health research emerged 2015-2024 AFTER approval
• Synthetic chemical, not natural: Created through chemical modification; structurally different from natural cellulose
• Gut barrier damage: Acts like a detergent, disrupting the protective mucus layer
• Dysbiosis at modest doses: Even 15g daily for 11 days causes microbiota damage in humans
• IBD risk: Associated with inflammatory bowel disease in large cohort study
• Cancer links: Animal studies show cancer promotion; large human cohort study found associations
• Metabolic dysfunction: Promotes obesity and metabolic syndrome
• Mechanism understood: Research clearly identifies how CMC damages gut health through mucus layer disruption
• Regulatory lag: EFSA assessments focus on toxic element contaminants, not the biological effects evidence that emerged post-approval
Practical recommendation: CMC is one of the additives most recommended for avoidance by those prioritizing gut health. Unlike truly safe additives like citric acid or pectin, CMC has demonstrated biological harm in rigorous human trials and large cohort studies. The mechanism of damage is understood—it disrupts the protective gut barrier. For people with existing IBD, IBS, or sensitivity, CMC should be strictly avoided. For the general population, reducing consumption of processed foods containing CMC benefits long-term gut health, microbiota diversity, and potentially reduces cancer risk. Choose products with natural thickeners (pectin, guar gum, locust bean gum) instead when possible.