What is E621 (MSG/Monosodium Glutamate)?
Complete guide to this controversial flavor enhancer with emerging evidence of neurological, cardiovascular, and metabolic health risks
The Quick Answer
E621 is monosodium glutamate (MSG)—the sodium salt of the amino acid glutamic acid, used as a flavor enhancer in thousands of processed foods. It works by stimulating umami (savory) taste receptors on the tongue, making food taste more flavorful and meaty without adding calories.
While regulatory authorities approve MSG as safe, independent research identifies significant health concerns that regulatory approval doesn’t adequately address. MSG has been independently classified as ORANGE—SOME CONCERNS by food safety evaluators—a middle-ground classification reflecting that while some people tolerate it fine, emerging evidence of neurological, cardiovascular, metabolic, and reproductive effects warrants caution, particularly for vulnerable populations.
Unlike truly safe additives like citric acid or pectin, MSG is a controversial excitotoxin that some research suggests may cause brain cell damage, obesity, insulin resistance, heart disease, and reproductive harm—concerns that regulatory approval standards may not adequately capture.
📌 Quick Facts
- Category: Flavor enhancer (umami taste booster), amino acid salt
- Composition: Sodium salt of L-glutamic acid (an amino acid)
- Found in: Instant noodles, snack foods, soups, sauces, processed meats, restaurant seasonings
- Safety Status: FDA GRAS approved; EFSA ADI 30 mg/kg bw/day; JECFA ADI “Not Specified”—but MODERATE controversy
- Controversy Level: ORANGE—SOME CONCERNS (independent rating)
- Emerging Concerns: Excitotoxicity, neurological damage, obesity, cardiovascular disease, reproductive effects
- Sodium Content: ~12% sodium; contributes to dietary sodium intake
- Symptom Complex: MSG symptom complex (MSC) affects <1% but includes headaches, flushing, palpitations, muscle aches
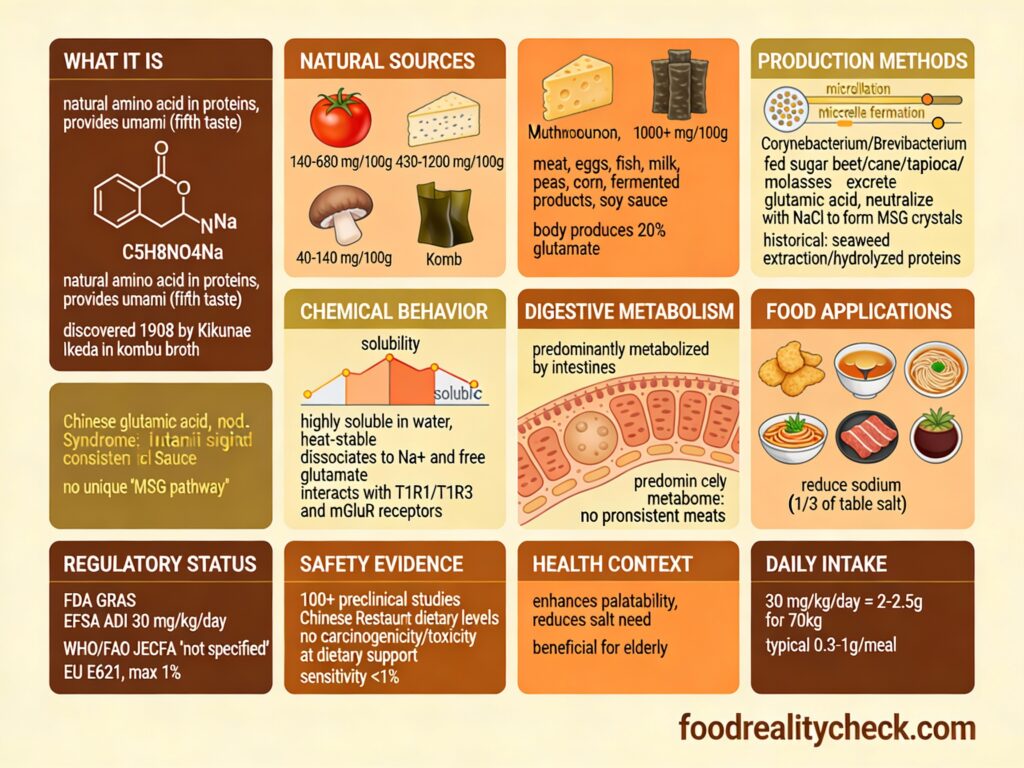
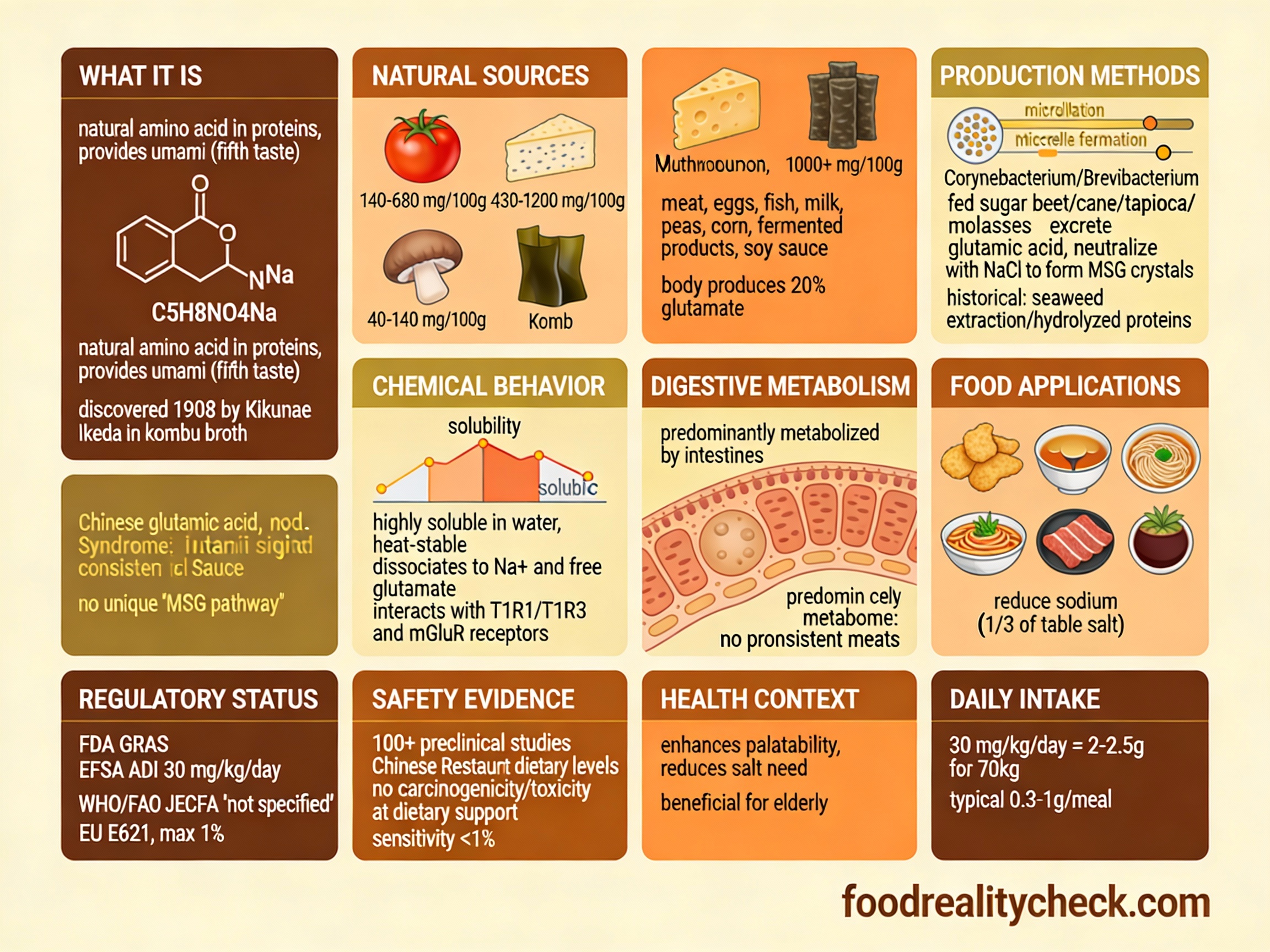
What Exactly Is Monosodium Glutamate?
Monosodium glutamate (MSG) is the sodium salt of glutamic acid, a naturally occurring non-essential amino acid. When dissolved in food or water, it dissociates into sodium ions and free glutamate, which binds to umami taste receptors on the tongue.
Chemical composition: The molecular formula is C₅H₈NO₄Na. Structurally, it’s glutamic acid (an amino acid normally produced by your body and found in proteins) neutralized with sodium.
In simple terms: It’s a salt of an amino acid that makes food taste savory and meaty. Your body produces glutamic acid naturally, and you consume it in protein-containing foods. MSG provides concentrated glutamic acid as a flavor enhancer.
Key properties:
• Umami taste booster: Stimulates specific taste receptors (T1R1/T1R3, mGluR variants) that detect savory taste
• Highly soluble: Dissolves easily in water and foods
• Heat stable: Maintains function during cooking
• Synergistic with nucleotides: Works powerfully with disodium inosinate (E631) and disodium guanylate (E627)—5-8 times more effective together
• Excitatory amino acid: Activates neural glutamate receptors; at high concentrations can cause excitotoxicity
• Sodium-containing: Contributes to dietary sodium intake
• BBB permeability concerns: Some research suggests MSG may cross the blood-brain barrier more readily than ingested glutamate from whole foods
⚠️ Critical Distinction: Natural vs Added Glutamate
This is central to understanding MSG’s risks versus dietary glutamate:
• Natural glutamate in foods: Bound in proteins; released slowly during digestion; often not fully bioavailable
• MSG added as flavor enhancer: Free, unbound glutamate in concentrated form; rapidly absorbed; spikes blood glutamate levels
• The difference matters: Concentrated free glutamate (MSG) has different pharmacological effects than glutamate from whole foods. It can saturate glutamate receptors and potentially cause excitotoxicity—neuronal damage from excessive glutamate receptor stimulation.
Where You’ll Find E621
MSG appears in thousands of processed foods, particularly Asian and fast foods:
• Instant ramen and noodle products
• Snack foods (crisps, chips, savory mixes)
• Instant soups and soup packets
• Bouillon cubes and stock cubes
• Processed meats and deli meats
• Cured meats and sausages
• Frozen meals and ready meals
• Sauces and gravy mixes
• Condiments (especially Asian brands)
• Fast food restaurant seasonings
• Canned soups and broths
• Chinese restaurant cooking
• Seasoning blends and spice mixes
• Pet foods
• Some low-sodium products (MSG used to replace salt while maintaining flavor)
How Is MSG Produced?
Modern MSG production uses bacterial fermentation:
Step 1: Fermentation Substrate
A carbohydrate source (sugar beet, sugar cane, tapioca starch, or molasses) is selected as the fermentation substrate.
Step 2: Bacterial Culture
Selected bacterial strains (Corynebacterium glutamicum or Brevibacterium species) are inoculated into the carbohydrate medium under controlled fermentation conditions (temperature, pH, aeration).
Step 3: Glutamic Acid Production
The bacteria metabolize the carbohydrates and excrete glutamic acid into the fermentation broth. This is a naturally occurring metabolic process—the bacteria produce glutamic acid as part of normal metabolism.
Step 4: Recovery & Purification
Glutamic acid is extracted from the fermentation broth through precipitation, separation, and purification.
Step 5: Neutralization
The glutamic acid is neutralized with sodium hydroxide to create sodium glutamate (monosodium glutamate):
Glutamic acid + Sodium hydroxide → Monosodium glutamate + Water
Step 6: Crystallization & Drying
The product is crystallized into white crystals and dried to create the food-grade powder.
Step 7: Quality Control
Tested for purity, sodium content, and microbiological safety before approval for food use.
Functions of E621 in Food
MSG serves one primary function:
As a flavor enhancer (umami booster): Stimulates umami taste receptors to enhance savory perception. A small amount makes food taste more flavorful, meaty, and satisfying. Particularly powerful when combined with nucleotide additives (E631, E627), where together they create a synergistic effect 5-8 times more potent than either alone.
Secondary benefits to manufacturers:
• Allows reduction of added salt while maintaining flavor appeal
• Masks off-flavors or unfamiliar tastes in processed foods
• Creates addictive quality encouraging repeat consumption
• Extremely cost-effective; small amounts achieve major flavor impact
Is E621 Safe? The Regulatory-Research Disconnect
MSG is approved as safe by regulatory authorities but rated ORANGE—SOME CONCERNS by independent food safety evaluators due to emerging health evidence that regulatory approval doesn’t adequately address.
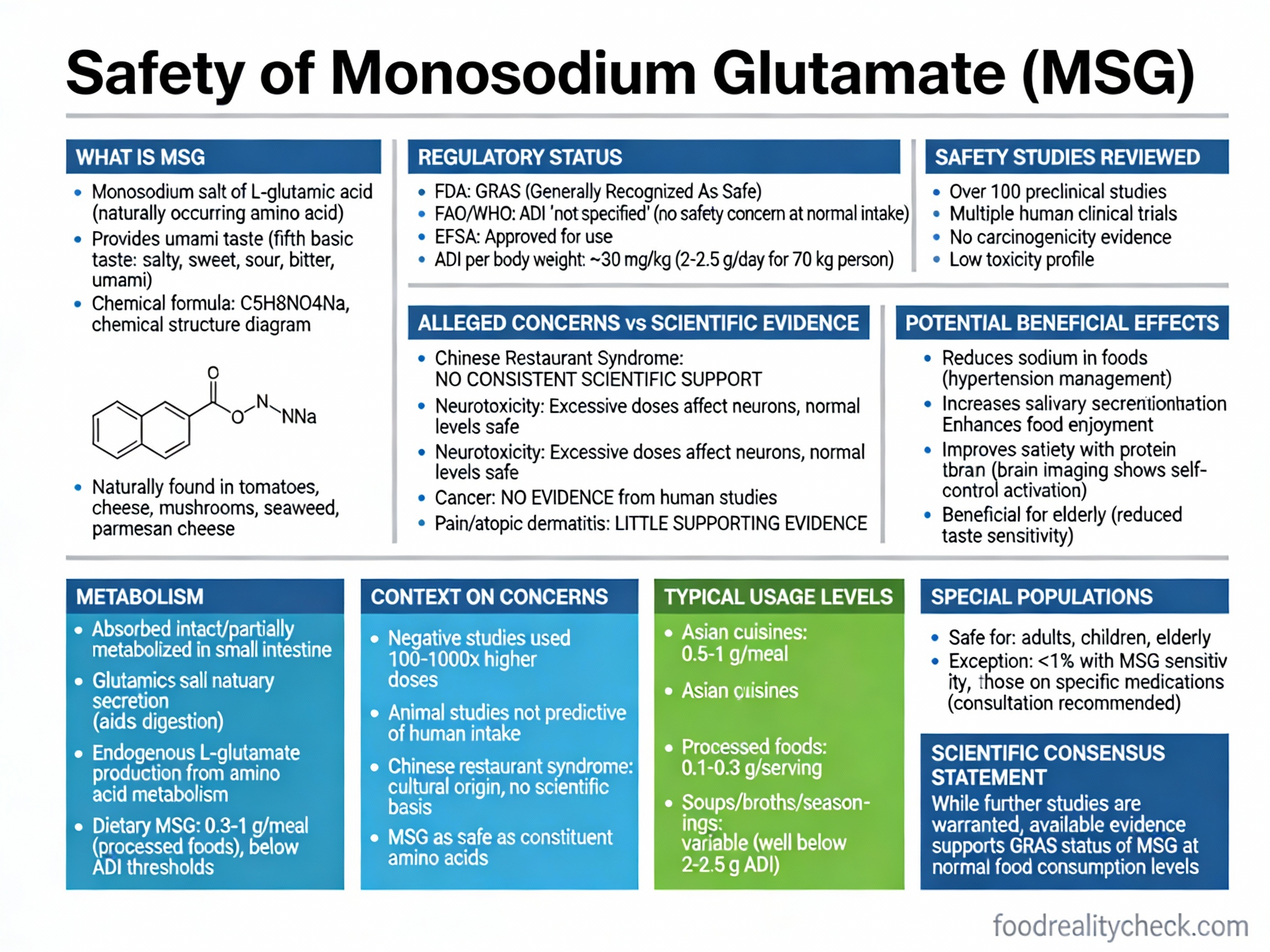
Regulatory Position (Approval-Based):
• FDA: GRAS (Generally Recognized As Safe); approved for use
• EFSA: ADI (Acceptable Daily Intake) set at 30 mg/kg bodyweight/day (as glutamic acid); some population groups may exceed this from high-consumption diets
• JECFA (WHO/FAO): ADI “Not Specified” (indicating no safety concerns at good manufacturing practice levels)
• Position: Safe at approved use levels and typical consumption
Independent Food Safety Rating (Research-Based):
Food safety evaluators classify MSG as ORANGE—SOME CONCERNS rather than GREEN—SAFE, specifically because:
1. Exceedance potential: EFSA estimated some high-consumption populations could exceed the 30 mg/kg ADI
2. Consumer reports of sensitivity: Documented MSG symptom complex (headaches, flushing, palpitations, muscle aches) in susceptible individuals
3. Emerging neurological research: Studies linking MSG to neurodegenerative disease, brain damage, and neurological dysfunction
4. Metabolic/cardiovascular concerns: Research showing associations with obesity, insulin resistance, and cardiovascular damage
Documented Health Concerns from Research:
Neurological Effects:
• Excitotoxicity: At high concentrations, glutamate causes excessive stimulation of neural glutamate receptors, leading to neuronal cell death
• Developmental neurotoxicity: Animal studies show neonatal MSG exposure causes permanent brain damage, particularly in the hypothalamus
• Neurodegenerative disease risk: Research links MSG consumption to increased risk of Parkinson’s disease, Alzheimer’s disease, Huntington’s disease, and other neurodegenerative conditions
• Cognitive impairment: Studies show impaired memory, learning, and cognition
• Behavioral disorders: Links to depression, anxiety, and psychiatric effects
Metabolic & Cardiovascular Effects:
• Obesity: MSG-induced obesity in animal models; promotes weight gain despite no caloric content
• Insulin resistance: Impairs glucose tolerance and insulin sensitivity; increases type 2 diabetes risk
• Cardiovascular damage: Studies show MSG causes dyslipidemia (abnormal lipid profile), oxidative stress, inflammatory response, and hepatic-cardiac tissue damage
• Hypertension: High-dose MSG significantly elevates systolic blood pressure
• Cardiac dysfunction: Impairs cardiac autonomic function; increases arterial pressure; causes myocardial damage
Reproductive Effects:
• Ovarian damage: Studies show MSG causes ovarian cell vacuolations, basement membrane changes, and cellular hypertrophy
• Reproductive dysfunction: Links to infertility and reproductive malfunctions
• Developmental toxicity: Maternal MSG consumption during pregnancy associated with offspring abnormalities
MSG Symptom Complex (MSC):
• Prevalence: Affects less than 1% of general population
• Symptoms: Headache, flushing, dizziness, numbness, tingling, muscle tightness, palpitations, chest pain, nausea, weakness
• Onset: Typically 15-30 minutes after consumption
• Mechanism: Not definitively understood; may involve neural sensitivity or vasodilation effects
• Controversy: Some studies in blinded trials fail to reproduce symptoms; others show real effects in sensitive individuals
⚠️ Key Concern: Regulatory Approval Doesn’t Mean Proven Safe
MSG’s EFSA ADI was set in 2013—12 years ago. Since then, extensive research has identified:
• Neurological damage from glutamate excitotoxicity
• Cardiovascular damage and oxidative stress
• Metabolic disruption and obesity promotion
• Reproductive system effects
• Potential links to neurodegenerative diseases
These findings suggest the regulatory safety assessment may not adequately capture real-world health risks. The fact that regulatory agencies set an ADI doesn’t mean the additive is proven harmless—it means it’s approved at specific use levels based on older safety standards.
Health Effects & Side Effects
Acute effects (from MSG sensitivity/MSG symptom complex):
• Headaches and migraines
• Flushing and skin redness
• Palpitations and chest pain
• Tingling, numbness, and paresthesia
• Muscle tightness and aches
• Nausea and dizziness
• Weakness and lethargy
• Shortness of breath
• In severe cases: loss of consciousness
Chronic effects (from regular consumption):
• Oxidative stress and inflammation
• Neurological changes and potential neurodegeneration
• Weight gain and obesity
• Insulin resistance and impaired glucose control
• Cardiovascular damage (myocardial damage, dyslipidemia)
• Reproductive dysfunction
• Hepatic (liver) damage
• Impaired cognitive function and memory
• Increased risk of neurodegenerative diseases
High-risk populations:
• Those with existing MSG symptom sensitivity
• Individuals with migraine disorders (MSG may trigger attacks)
• Those with asthma (some studies show potential triggers)
• People with metabolic syndrome or obesity
• Those with cardiovascular disease
• Individuals with neurological conditions
• Pregnant women and developing fetuses
• Neonates and young children (developing brains more vulnerable)
The Bottom Line
E621 (monosodium glutamate) is approved as safe by regulatory authorities but rated ORANGE—SOME CONCERNS by independent food safety evaluators due to emerging evidence of neurological, cardiovascular, metabolic, and reproductive health risks. Unlike clearly safe additives like citric acid or pectin, MSG sits in a controversial middle ground where regulatory approval and independent research diverge significantly.
Key takeaways:
• Regulatory approval but moderate controversy: FDA GRAS; EFSA ADI 30 mg/kg/day; but rated ORANGE—SOME CONCERNS independently
• Excitotoxicity concern: At high concentrations, can cause neuronal damage through excessive glutamate receptor stimulation
• Emerging neurological risks: Research links to neurodegenerative diseases, brain damage, cognitive impairment
• Cardiovascular damage: Studies show oxidative stress, myocardial damage, dyslipidemia, hypertension
• Metabolic disruption: Promotes obesity, insulin resistance, and glucose intolerance despite zero calories
• Reproductive effects: Evidence of ovarian damage and developmental toxicity
• MSG symptom complex: Documented sensitivity in <1%, but real effects including headaches, palpitations, neurological symptoms
• Synergistic danger: When combined with nucleotide additives (E631, E627), effects multiply 5-8 fold
• Regulatory gap: Last comprehensive EFSA re-evaluation was 2013; emerging research suggests safety reassessment is overdue
Practical recommendation: MSG is not in the category of clearly safe additives like citric acid or pectin. While it’s approved for use, mounting evidence of neurological, cardiovascular, and metabolic effects warrants significant caution. Particularly vulnerable populations (pregnant women, children, those with neurological or metabolic conditions) should minimize consumption. If you’re a regular consumer of processed foods, instant noodles, snacks, or fast food (which are often MSG-laden), reducing this consumption benefits health in multiple ways. Those with migraine disorders or who suspect MSG sensitivity should strictly avoid it. The regulatory approval doesn’t mean MSG is proven safe—only that it’s approved at certain use levels based on older safety standards.