The future of protein production—cultivated in bioreactors, not on farms.
The Overview
Lab-grown meat, also called cultivated or cultured meat, is genuine animal tissue produced by growing animal cells in controlled laboratory environments rather than raising and slaughtering livestock.
The process leverages cell biology and tissue engineering to transform a few cells into pounds of edible meat in weeks, not years.
Here’s exactly how cells become steak, burger, or chicken through sophisticated biotech.
🥘 Core Components
• Stem cells or muscle satellite cells (from animal biopsy)
• Culture media (nutrients, growth factors, hormones)
• Bioreactors (large fermentation tanks)
• Scaffolding materials (edible or biodegradable support structures)
• Microcarriers (small beads for cell attachment)
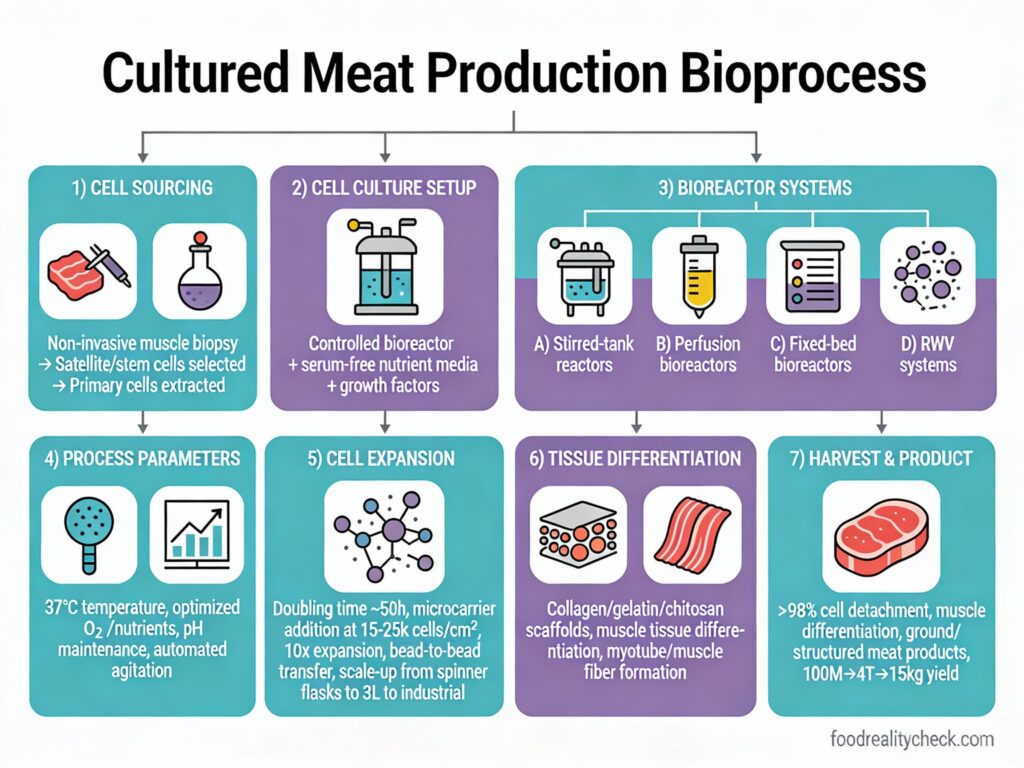
Step 1: Cell Sourcing & Banking
A small biopsy is taken from a live animal (typically cow, chicken, or fish) without killing it—sometimes from muscle tissue, other times from an egg cell.
Scientists isolate stem cells or muscle satellite cells (myosatellite cells) that have the ability to proliferate indefinitely and differentiate into muscle or fat cells.
A “master cell bank” is created and cryogenically frozen, providing a stable, renewable source of starter cells for every batch of meat production.
Step 2: Cell Line Development & Quality Control
Isolated cells are screened and tested for their ability to grow rapidly, remain viable in culture, and differentiate into desired cell types.
Cells are tested for contamination, genetic stability, and growth characteristics before being approved for scale-up.
This quality assurance step ensures every production run starts with healthy, consistent cells.
Step 3: Initial Culture & Expansion (Lab Scale)
Thawed cells are placed in petri dishes or small culture vessels containing nutrient-rich growth medium—a cocktail of amino acids, vitamins, glucose, hormones, and growth factors that mimic conditions inside an animal’s body.
Under controlled temperature (37°C) and pH (7.2-7.4), cells divide and multiply exponentially.
After 3-5 days, cell populations grow from thousands to millions without any genetic modification.
Step 4: Scaling to Bioreactors
Once cell populations are sufficient, cells are transferred to large bioreactors—industrial-scale fermentation tanks ranging from 100 liters to 15,000+ liters in capacity.
Bioreactors provide automated control of temperature, pH, oxygen, CO2, and nutrient levels—conditions optimized for massive cell expansion.
Modern bioreactors use microcarriers (tiny beads coated with cell-adhesion proteins) or suspension systems where cells grow as aggregates, maximizing surface area for cell attachment and growth.
Step 5: Proliferation Phase (Cell Expansion)
Inside the bioreactor, cells undergo exponential growth over 5-10 days, multiplying from millions to billions.
Fed-batch or continuous bioreactor modes add fresh growth medium periodically while removing waste products (ammonia, lactate) that inhibit cell growth.
Microcarrier beads are progressively added to increase surface area—cells naturally migrate from fully colonized beads to new ones in a process called bead-to-bead transfer.
Differentiation: Building the Three Tissues of Meat
Step 6: Medium Switching & Differentiation Signals
Once sufficient cells are generated, the growth medium is switched to differentiation medium—a reformulated nutrient mixture containing different growth factors and hormones.
These signals trigger stem cells to specialize into one of three cell types: myoblasts (muscle), adipocytes (fat), or fibroblasts (connective tissue).
Sequential differentiation ensures spatial organization—muscle cells form first, then fat cells are induced to proliferate without competing for resources.
Step 7: Tissue Maturation & Muscle Fiber Formation
Myoblasts (immature muscle cells) fuse together to form long, multinucleated muscle fibers, mimicking the structure of real meat.
Gentle mechanical stimulation (stretching or electrical pulses in advanced bioreactors) helps muscle fibers organize and mature.
Over 1-2 weeks, these fibers develop contractility and the characteristic texture of muscle tissue.
Step 8: Scaffolding & Structure Formation
To create a structured meat product (burger vs. steak), differentiated cells are organized onto edible scaffolds—3D structures made from materials like plant proteins, alginate, or biodegradable polymers.
Scaffolds provide a framework for cells to attach to and guide their organization into a meat-like structure.
For ground meat (burgers), minimal scaffolding is needed; for whole cuts (steaks), complex 3D structures replicate the layered architecture of real meat.
Step 9: 3D Bioprinting (Advanced Method)
Cutting-edge facilities use 3D bioprinting—depositing cells layer-by-layer in precise patterns, similar to inkjet printing.
This technology allows specification of muscle-to-fat ratios and spatial organization identical to traditional meat cuts.
Printed structures are matured in perfusion bioreactors that pump nutrients through the tissue, ensuring cells in the center receive adequate oxygen and nutrients.
Harvest, Processing, and Product Formation
Step 10: Cell Harvesting
Once tissue maturation is complete (typically 2-8 weeks from initial inoculation), the cultured biomass is harvested from the bioreactor.
Cells are gently detached from microcarriers or scaffolds using enzymatic or mechanical methods.
The harvested cell mass contains muscle, fat, and connective tissue—the three components needed for a complete meat product.
Step 11: Food Processing & Texturization
The harvested biomass undergoes conventional food processing techniques: grinding, shaping, seasoning, and cooking if needed.
For ground meat products, cells are ground into a texture resembling ground beef, sausage, or minced meat.
For whole-cut steaks, the bioprinted or scaffolded structure is carefully processed to maintain its integrity while achieving the desired texture and appearance.
Step 12: Quality & Safety Testing
Every batch undergoes rigorous microbial testing, nutritional analysis, and safety verification following food industry standards (ISO 22000, FSMA compliance).
Products are tested for pathogens, heavy metals, and nutritional consistency before release.
Regulatory frameworks (FDA in the US, FSA in the UK, EFSA in EU) oversee approval, with Singapore becoming the first country to approve cultured meat for human consumption in 2020.
Step 13: Packaging & Distribution
Finished meat products are packaged in refrigerated or modified-atmosphere packaging similar to conventional meat.
Product shelf-life is maintained through cold storage (2-4°C) and resembles fresh meat’s typical shelf-life of 1-2 weeks.
Products are distributed to retailers or restaurants through standard cold-chain logistics.
Why This Process?
Cultivated meat uses 95% less land than conventional beef production and requires 78% less water while producing identical nutritional profiles.
The process eliminates animal slaughter, improves food safety (no zoonotic pathogens), and dramatically reduces greenhouse gas emissions (up to 96% lower than conventional beef).
Scale-up is rapid—cell doubling occurs every 24-48 hours in optimized bioreactors, producing finished product in 2-8 weeks versus 2-3 years for conventional cattle.
Current Challenges & Additives
Most cultured meat currently relies on:
• Plant-derived growth factors & hormones – to stimulate cell proliferation
• Serum components (being replaced by plant-based alternatives) – to provide growth support
• Scaffolding materials (edible plant proteins or biodegradable polymers) – for structure
• Standard food additives – for taste, preservation, and food safety
Key challenges include the high cost of growth media, scaling production to cost-competitive volumes, and regulatory acceptance in global markets.
Current products are already approved in Singapore and approved for pet food in the UK as of 2024, with human consumption approvals pending in the US and Europe.
The Bottom Line
Lab-grown meat represents a paradigm shift in protein production—from raising animals to cultivating cells in bioreactors.
The process combines cell biology, tissue engineering, and bioprocess automation to create genuine animal meat without the environmental impact or ethical concerns of traditional livestock farming.
Now you understand exactly how a single cell sample becomes a steak, burger, or chicken breast through 2-8 weeks of controlled cellular growth and differentiation.