What is E201?
Complete guide to understanding E201 (Sodium Sorbate) in your food
The Quick Answer
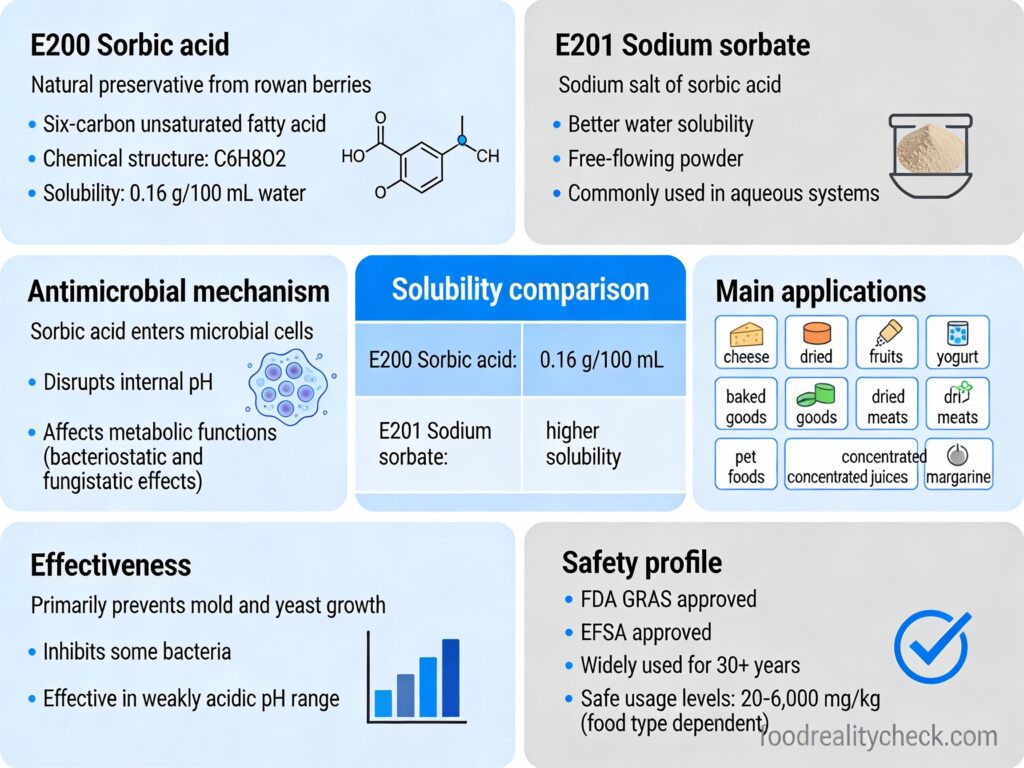
E201 is sodium sorbate—the sodium salt of sorbic acid, a preservative that inhibits growth of molds, yeasts, and some bacteria in foods.
CRITICAL REGULATORY DISTINCTION: E201 is PROHIBITED for food use in the European Union due to potential genotoxic (DNA-damaging) effects. It is approved only in the United States, Canada, and some other non-EU countries for limited applications (cheese, fruit preserves, margarine). Unlike E200 (sorbic acid) which is EU-approved and considered safe, E201 carries genotoxicity concerns that led to its EU ban in 1998.
📌 Quick Facts
- Category: Synthetic preservative (antimicrobial); sodium salt of sorbic acid
- Chemical Formula: C₆H₇NaO₂
- Appearance: Unstable white crystalline powder; highly water-soluble (unlike E200)
- EU Status: PROHIBITED since 1998 due to potential genotoxic effects
- USA/Canada Status: GRAS approved; permitted for cheese, fruit preserves, margarine
- Found in (Outside EU): Cheese, fruit jams, margarine, some beverages
- Key Difference from E200: While sorbic acid (E200) is approved in EU with no major concerns, E201 (sodium salt) is banned due to genotoxicity concerns
- Safety Status: GENOTOXIC effects documented in vitro; human risk assessment inconclusive but precautionary ban implemented

What Exactly Is It?
E201 is sodium sorbate—the sodium salt of sorbic acid, created by neutralizing sorbic acid with sodium hydroxide.
Chemical formula: C₆H₇NaO₂; molecular weight 134.11.
E201 appears as an unstable white crystalline powder. Unlike sorbic acid (E200), which has poor water solubility, sodium sorbate is highly water-soluble, making it suitable for aqueous food systems.
Production: Sodium sorbate is produced by neutralizing sorbic acid (itself synthesized from crotonaldehyde and ketene) with sodium hydroxide or sodium carbonate, resulting in the sodium salt.
Mechanism: Like sorbic acid, sodium sorbate functions as a preservative by inhibiting growth of molds, yeasts, and some bacteria. The mechanism is thought to involve disruption of microbial metabolic pathways. Most effective at pH below 6.5 (acidic conditions).
Geographic Regulatory Status – CRITICAL DISTINCTION
This is the most important point about E201: regulatory status varies dramatically by region, reflecting different assessments of genotoxicity concerns.
European Union: PROHIBITED since 1998 due to suspected mutagenic (genotoxic) effects. Not permitted in any EU member states. German approval was revoked in 1998; pan-EU ban followed due to genotoxicity concerns.
United States: GRAS (Generally Recognized As Safe) approved for cheese, fruit butter, fruit jelly, fruit preserves, jams, and margarine.
Canada: Approved for limited applications (cheese, fruit preserves, margarine).
Australia & New Zealand: Regulatory status varies; not universally approved.
Where You’ll Find It (Outside EU)
In countries where E201 is permitted:
• Cheese and cheese products
• Fruit jams, jellies, and preserves
• Margarine and butter spreads
• Candied fruits
• Some baked goods
• Wine and soft drinks (in some countries)
• Pickled vegetables
• Processed cheese spreads
• Some processed meats
E201 is much less common than E200 (sorbic acid) because it has been banned in the EU (world’s largest food market) and requires caution in other regions due to genotoxicity concerns.
🛑 RED SAFETY RATING – GENOTOXICITY CONCERNS: E201 has:
• Documented genotoxic effects in vitro: 2012 study in human lymphocytes showed increased chromosome aberrations, sister-chromatid exchanges, and micronucleus formation at high concentrations
• EU precautionary ban: Banned 1998 due to suspicion of mutagenic effects; not lifted despite decades of use in other countries
• In vitro DNA damage: Research shows DNA damage in isolated human lymphocytes even at lower concentrations
• Micronucleus induction: Increased micronucleus frequency implies potential cancer risk in humans
• Conflicting genotoxicity data: Some animal studies negative; human cell studies show DNA damage, particularly at highest test concentrations
• Risk-benefit assessment unfavorable: EU determined genotoxicity concerns outweigh preservative benefits; alternative E200 (sorbic acid) available without genotoxicity concerns
This is markedly different from E200 (sorbic acid), which is approved in the EU without genotoxicity concerns. The key distinction is the sodium salt vs. free acid form.
Is It Safe?
Safety assessment of E201 is fundamentally complicated by regulatory disagreement: The EU considers it unsafe and banned it in 1998; the USA approves it as GRAS. This represents a major regulatory split on the same chemical.
Available safety data is mixed and concerning:
In Vitro Genotoxicity (Concerning): A 2012 study in human peripheral blood lymphocytes found that sodium sorbate significantly increased chromosome aberrations, sister-chromatid exchanges, and micronucleus formation, particularly at concentrations of 400-800 μg/ml. While these concentrations are higher than typical food exposure, the findings are still concerning.
In Vivo Animal Studies (Mixed): Some in vivo animal studies in Chinese hamster and mouse bone marrow cells showed negative results for genotoxicity (SCEs tests), while other studies showed positive results. This inconsistency is problematic for risk assessment.
EU Risk Assessment (Precautionary): The EU concluded in 1998 that suspected mutagenic effects were sufficient grounds for banning E201, even without definitive human evidence of harm. This reflects a precautionary principle: when in doubt about genotoxic potential, remove the additive if alternatives exist.
USA GRAS Designation (Permissive): Despite genotoxicity studies, the FDA maintains GRAS status, focusing on lack of documented adverse events in human consumers rather than in vitro findings.
What Are The Health Concerns?
The primary health concern with E201 is potential genotoxicity (DNA damage). Secondary concerns include allergic reactions (shared with all sorbates).
Primary Concern—Genotoxicity: In vitro studies in human lymphocytes show DNA damage, chromosome aberrations, and micronucleus formation, particularly at high concentrations. Micronucleus formation is associated with cancer risk in humans. This concern is serious enough to have triggered EU prohibition.
Human Evidence Gap: Despite decades of use in USA and Canada, no documented cases of cancer specifically attributed to E201 exist, nor clear dose-related adverse events in humans. However, absence of documented harm does not equal safety guarantee, particularly for genotoxic agents where effects may take years to manifest.
Secondary Concern—Allergic Reactions (Rare): Like all sorbates, E201 may trigger pseudo-allergic reactions (hypersensitivity) in sensitive individuals, though this is rare. Reactions can include hives, dermatitis, or urticaria.
Behavioral Concerns (Controversial): Some sources suggest E201 may cause behavioral problems, though this is speculative and not confirmed by authoritative bodies.
Instability Issue: Sodium sorbate is chemically unstable and can oxidize, potentially producing harmful degradation products. This is a manufacturing/storage quality issue, not an intrinsic toxicity concern.
Why the EU Ban?
The 1998 EU ban of E201 reflects a risk-benefit calculation:
• In vitro genotoxicity data: Multiple studies show DNA damage in human cells, raising concern about carcinogenic potential.
• Alternative available: Sorbic acid (E200) provides identical preservative function without documented genotoxicity concerns. When safe alternatives exist, precautionary principle suggests removing potentially hazardous additives.
• Burden of proof: EU requires additives to prove safety; genotoxicity concerns shifted burden away from E201.
• Precautionary principle: In absence of definitive human safety data for a genotoxic agent, EU chose to err on the side of caution.
Why USA Still Approves It?
FDA’s GRAS designation despite genotoxicity studies reflects:
• Different regulatory philosophy: USA focuses on documented adverse events in human consumers rather than in vitro findings alone.
• Decades of use without incident: Lack of documented cancer clusters or health problems attributed to E201 in USA/Canada consumers.
• Dose considerations: In vitro genotoxicity observed at concentrations (400-800 μg/ml) potentially higher than typical food exposure.
• Conservative approach: FDA has historically been more permissive with additives that lack clear human adverse events, even when in vitro data raises concerns.
Natural Alternatives
Want to avoid E201?
• Sorbic acid (E200): Same preservative function, EU-approved, no documented genotoxicity concerns—the preferred alternative
• Potassium sorbate (E202): Alternative sorbate salt, EU-approved, no documented genotoxicity concerns
• Benzoic acid (E210): Alternative synthetic preservative, approved in most regions
• Ascorbic acid (E300/Vitamin C): Natural antioxidant, less effective as preservative
• Salt-based preservation: Traditional method for cured meats and pickled vegetables
• Refrigeration/Freezing: Alternative preservation methods
The Bottom Line
E201 (Sodium Sorbate) is a preservative that is PROHIBITED in the European Union due to potential genotoxic (DNA-damaging) effects, while remaining GRAS-approved in the USA and Canada. This represents a significant regulatory disagreement about the same chemical. In vitro studies document DNA damage in human cells, which triggered the EU’s precautionary ban. However, decades of use in non-EU countries without documented human adverse events maintains FDA’s GRAS approval. This is fundamentally different from E200 (sorbic acid), which provides identical preservative function without genotoxicity concerns, making E200 the preferred choice.
EU Regulatory Decision (1998 Ban): Precautionary principle applied; genotoxicity concerns sufficient to ban when safe alternative (E200) exists.
Documented In Vitro Genotoxicity: 2012 study in human lymphocytes showed chromosome aberrations, sister-chromatid exchanges, and micronucleus formation, particularly at high concentrations.
Regulatory Split Reflects Different Philosophies: EU: precautionary (remove potentially genotoxic agents); USA: based on documented human evidence (GRAS because no adverse events documented).
Human Safety Data Gap: Despite in vitro concerns, no documented cases of cancer or serious health effects specifically attributed to E201 in human consumers exist. However, absence of evidence is not evidence of absence, particularly for carcinogenic effects with long latency periods.
Safe Alternative Available: Sorbic acid (E200) provides identical preservative function without genotoxicity concerns. This is why EU considers E201 unnecessary and chose to ban it.
Recommendation: E201 represents a case where regulatory bodies have reached different conclusions about the same additive. Given that:
(1) Genotoxicity documented in vitro,
(2) Safe alternatives (E200, E202) available,
(3) EU precautionary ban established,
most consumers should avoid E201 if possible. In the EU, it is already prohibited. In USA/Canada, consumers concerned about genotoxicity potential should prefer foods preserved with E200 (sorbic acid) or E202 (potassium sorbate) instead. This is one of the rare cases where a regulatory split reflects legitimate scientific debate rather than arbitrary difference.