What is E211?
Complete guide to understanding E211 (Sodium Benzoate) in your food
The Quick Answer
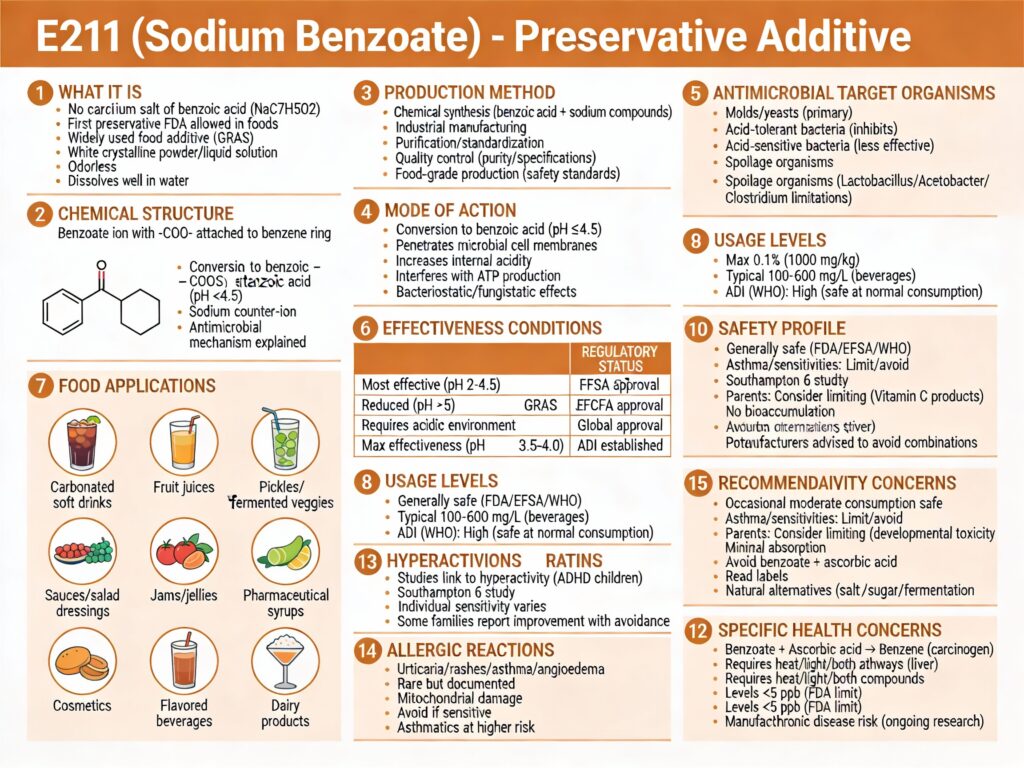
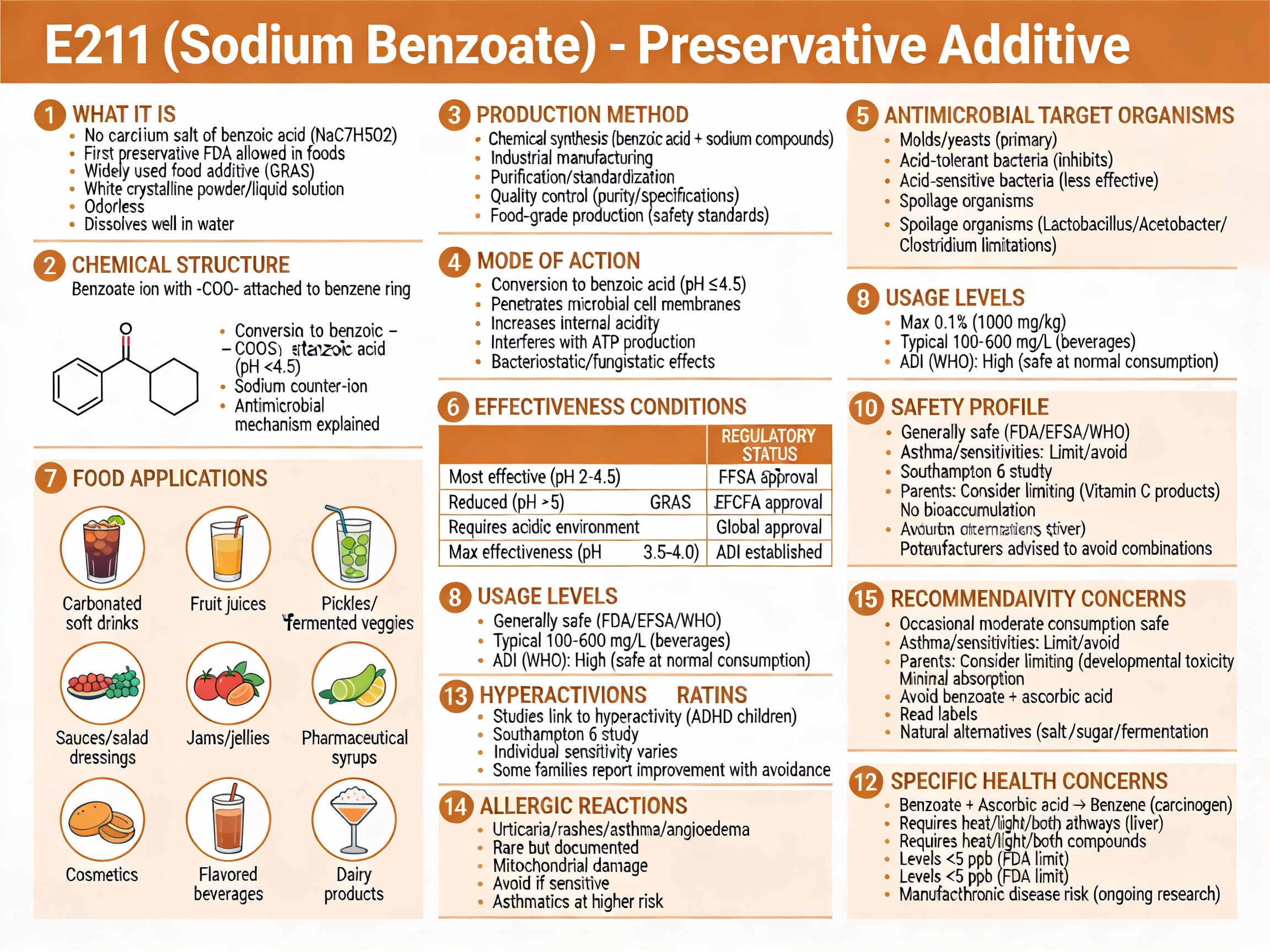
E211 is a synthetic preservative that prevents bacterial, mold, and yeast growth in acidic foods.
It’s used in soft drinks, juices, sauces, and pickles to extend shelf-life without refrigeration.
It’s approved globally but more controversial than E202, with emerging health concerns about benzene formation and hyperactivity.
📌 Quick Facts
- Category: Preservative (antimicrobial agent)
- Chemical Name: Sodium salt of benzoic acid (C₆H₅COONa)
- Found in: Soft drinks, juices, pickles, sauces, condiments, jams, medicines
- Safety: Generally Recognized as Safe (GRAS); approved but with emerging concerns
- ADI (EFSA/JECFA): 5 mg/kg body weight per day (expressed as benzoic acid)
- Maximum permitted: 0.1% by weight in most foods
What Exactly Is It?
E211 is a synthetic sodium salt created by neutralizing benzoic acid with sodium hydroxide.
Its chemical formula is C₆H₅COONa (sodium benzoate).
Benzoic acid occurs naturally in small amounts in berries (cranberries, blueberries, plums, apples), seafood, and dairy products, but E211 itself is entirely synthetically manufactured.
It appears as a white crystalline powder with no odor, highly soluble in water (making it ideal for liquid foods like soft drinks).
In acidic foods, sodium benzoate dissociates and converts into benzoic acid, the actual antimicrobial agent.
Where You’ll Find It
E211 appears in many common foods and beverages:
• Soft drinks and colas
• Fruit juices and juice concentrates
• Carbonated beverages
• Pickles and relishes
• Salad dressings and condiments
• Jams, jellies, and marmalades
• Sauces (tomato, soy, fish)
• Frozen yogurt toppings
• Medicines and pharmaceutical syrups (cough syrup)
• Cosmetics and personal care products
• Some bread and baked goods
• Maraschino cherries and preserved fruits
China is the world’s largest manufacturer of sodium benzoate, producing over 90,500 MT annually.
Why Do Food Companies Use It?
E211 serves one critical function: prevent bacterial, yeast, and mold growth in acidic foods.
It’s particularly effective in:
Soft drinks: Carbonic acid naturally makes sodas acidic (pH 2.5–4.0), ideal for E211 effectiveness. It prevents fermentation and shelf-stable storage at room temperature for months/years.
Fruit juices: Citric acid lowers pH, allowing E211 to prevent mold and wild yeast fermentation without heat pasteurization.
Pickles and relishes: Acetic acid (vinegar) creates an acidic environment where E211 effectively stops spoilage organisms.
Cost reduction: E211 allows manufacturers to reduce expensive preservation methods like heat treatment, sugar, or salt.
Long shelf-life without refrigeration: Particularly valuable for products exported globally or stored in environments without reliable refrigeration.
Is It Safe?
E211 is approved globally but carries more health concerns than E202 (potassium sorbate), with several controversial issues.
The FDA classifies sodium benzoate as “Generally Recognized as Safe” (GRAS) for use at up to 0.1% in food.
The EFSA re-evaluated sodium benzoate in 2016 and established an ADI of 5 mg/kg body weight per day (expressed as benzoic acid).
JECFA also set an ADI of 5 mg/kg per day.
To reach this ADI limit, an adult would need to consume approximately 350 mg of sodium benzoate daily—equivalent to roughly 70 cans of cola.
What Are The Health Concerns?
E211 has several documented health concerns, more significant than E202:
Benzene formation (carcinogen): When sodium benzoate combines with ascorbic acid (vitamin C) or erythorbic acid under heat or light, it transforms into benzene—a compound linked to cancer. The FDA acknowledges this risk and discourages such formulations. Manufacturers now typically avoid combining these ingredients.
Hyperactivity in children: Multiple studies (particularly the UK Southampton Study, 2007) linked sodium benzoate and other synthetic food colors to increased hyperactivity and behavioral problems in children. Some studies showed a dose-dependent relationship. The EFSA noted behavioral effects were “a cause for concern” but disagreed with regulatory action, citing inconsistent evidence. The debate continues.
Allergic reactions and hypersensitivity: A small percentage of people (estimated 0.5–1%) show hypersensitivity to sodium benzoate, manifesting as urticaria (hives), contact dermatitis, pruritus (itching), or rarely angioedema. Asthmatics may experience exacerbation.
Mutagenesis and oxidative stress (emerging concerns): Recent critical reviews note sodium benzoate may produce mutagenesis consequences (genetic damage), oxidative stress, and hormone disruption in laboratory studies. However, human studies are limited.
Liver and kidney concerns (high-dose animal studies): Studies in pregnant rats receiving high doses showed elevated ALT (liver enzyme), decreased bilirubin, and increased urea. However, these were extreme doses unrelated to food consumption.
Microbiome alteration: Like potassium sorbate, sodium benzoate may alter gut microbiota composition, though human clinical significance remains unclear.
Natural vs Synthetic Version
E211 is entirely synthetically manufactured.
While benzoic acid occurs naturally in berries and some foods, E211 sodium benzoate used in food additives is produced industrially by neutralizing benzoic acid (itself produced via partial oxidation of toluene with oxygen) with sodium hydroxide.
No commercial E211 is derived from natural sources; all is synthetic.
Natural Alternatives
Want to avoid E211?
Food companies sometimes use alternative preservatives:
• Potassium sorbate (E202) – widely used alternative, generally considered safer than E211
• Potassium benzoate (E212) – reduces sodium intake (used by PepsiCo in Pepsi)
• Calcium benzoate (E213) – alternative salt form
• Nisin (E234) – natural bacteriocin from lactic acid bacteria
• Natural fermentation – probiotics and lactic acid
• Ascorbic acid/vitamin C (E300) – natural antioxidant (though avoid combining with sodium benzoate)
• Heat treatment/pasteurization – thermal preservation
E202 (potassium sorbate) is increasingly replacing E211 in soft drinks and beverages due to fewer health concerns.
The Bottom Line
E211 is a widely used synthetic preservative that’s approved but more controversial than E202, with documented concerns about benzene formation (when combined with vitamin C), hyperactivity in children, and allergic reactions.
Regulatory disagreement: The FDA maintains E211 is safe; the EFSA acknowledged behavioral effects were “a cause for concern” but didn’t recommend restrictions; health agencies worldwide remain split on the severity of emerging concerns.
Benzene issue is most serious: The documented carcinogenic benzene formation when combined with vitamin C is the strongest health concern and the primary reason manufacturers have reformulated to avoid this combination.
Hyperactivity concerns are debated: Some studies link sodium benzoate to childhood hyperactivity; others dispute the finding. This remains scientifically unsettled.
If you want to minimize exposure: Avoid soft drinks and beverages combining sodium benzoate with vitamin C. Choose products with potassium sorbate (E202) instead, which has fewer documented concerns. Read labels carefully—check for both preservative and vitamin C co-existence.
For most people: Occasional consumption at approved levels is unlikely to cause harm. However, regular consumption of high quantities—particularly beverages combining sodium benzoate and vitamin C—warrants caution and reduction.