What is E224?
Complete guide to understanding E224 (Potassium Metabisulfite) in your food
The Quick Answer
E224 is potassium metabisulfite—a potassium salt of sulfurous acid that functions as a preservative, antioxidant, and bleaching agent in wines, beverages, dried fruits, and other foods by releasing sulfur dioxide to prevent spoilage, oxidation, and discoloration.
CRITICAL SAFETY WARNING: E224 is part of the sulfite family (E220-E228) which carries well-documented health risks, particularly for asthmatics. Clinical research confirms 3-10% of chronic asthmatics experience adverse respiratory reactions. Key documented concerns include: (1) bronchial constriction, wheezing, and respiratory distress in 3-10% of chronic asthmatics—with some experiencing life-threatening bronchospasm requiring hospitalization; (2) severe and life-threatening reactions documented in 5-10% of asthmatic patients; (3) steroid-dependent asthmatics at significantly elevated risk; (4) anaphylactic shock documented; (5) toxicity research showing E224 produces excessive free radicals and is toxic even at the lowest recommended ADI concentration (0.7 mg/kg) in animal studies; (6) EFSA ADI is temporary and pending re-evaluation due to uncertainties; (7) reactions do not respond to antihistamines; (8) preferred over sodium metabisulfite only because it does not add dietary sodium. E224 should be strictly avoided by all asthmatics, steroid-dependent patients, and those with documented sulfite sensitivity.
📌 Quick Facts
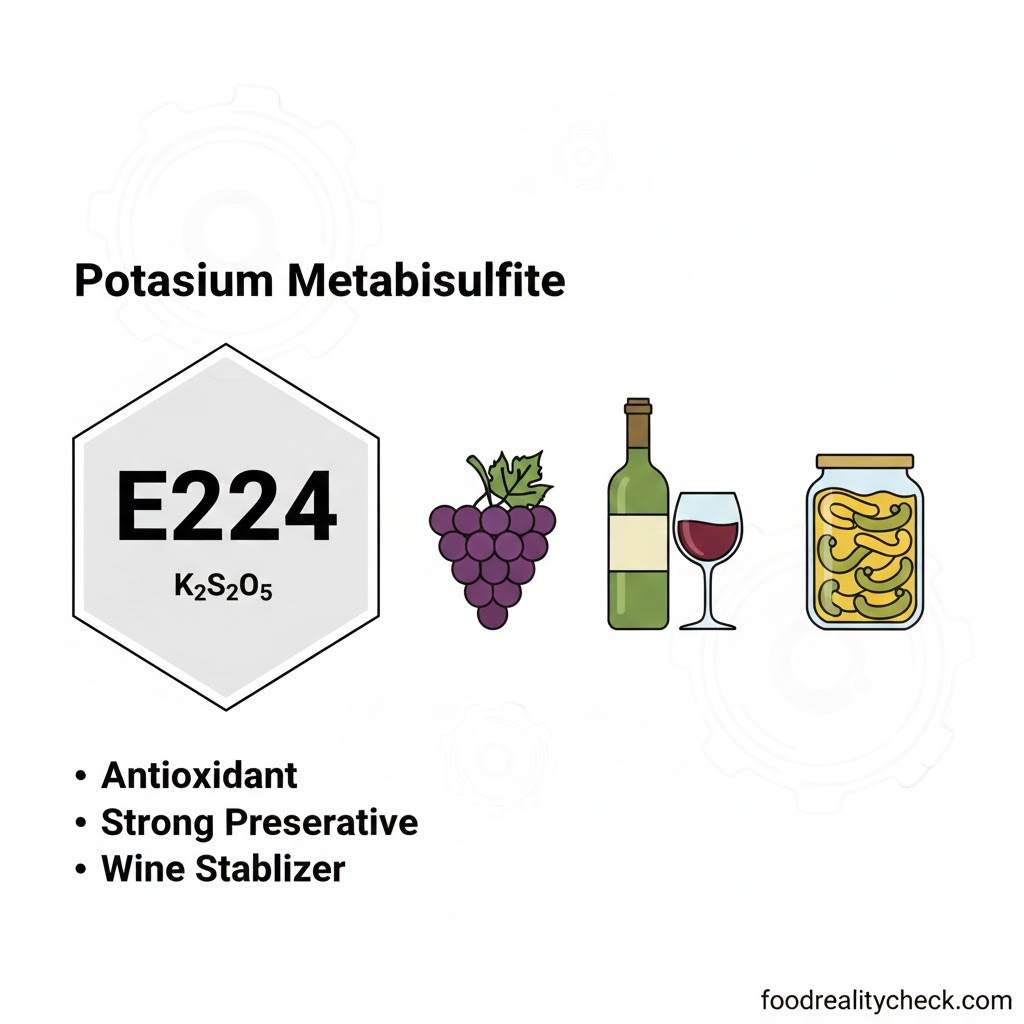
- Category: Synthetic preservative, antioxidant, bleaching agent; potassium salt of sulfurous acid
- Chemical Formula: K₂S₂O₅ (potassium pyrosulfite or potassium disulfite)
- Appearance: White crystalline powder; pungent odor of sulfur dioxide; unstable; releases SO₂ gas when dissolved
- Safety Status: FDA GRAS; EFSA approved; JECFA approved (with 2016+ safety concerns)
- ADI (Acceptable Daily Intake): 0.7 mg/kg body weight/day (as SO₂ equivalent; TEMPORARY, pending EFSA re-evaluation)
- Found in: Wine (major use), cider, beer, juice, dried fruits, dried vegetables, jams, pickles
- KEY CONCERN: 3-10% of chronic asthmatics experience adverse reactions; 5-10% may experience severe/life-threatening bronchospasm
- RESEARCH FINDING: Toxic at the lowest recommended ADI concentration; produces excessive free radicals (rat studies)
What Exactly Is It?
E224 is potassium metabisulfite—a potassium salt of sulfurous acid produced by combining sulfur dioxide with potassium carbonate or hydroxide, functioning as a reducing agent and antimicrobial preservative by releasing sulfur dioxide gas when dissolved.
Chemical formula: K₂S₂O₅ (also called potassium pyrosulfite or potassium disulfite); molecular weight 222.33 g/mol.
E224 appears as colorless or white crystalline powder with pungent sulfur dioxide odor. It is soluble in water (44.9 g/100 ml at 20°C; 250 g/L at 0°C), gradually oxidizing to potassium sulfate on air exposure. Aqueous pH 3.4-4.5 (acidic).
Critical chemical reaction: When dissolved in water, potassium metabisulfite dissociates and releases potassium bisulfite (KHSO₃) and sulfur dioxide (SO₂) gas:
K₂S₂O₅ + H₂O → 2 KHSO₃ → sulfurous acid + SO₂ gas
Production: SO₂ + K₂CO₃ (or KOH) → K₂S₂O₅ at 50-80°C, pH 4-7.5. Industrially produced; widely manufactured globally.
Mechanism: Released SO₂ gas and sulfurous acid in acidic conditions disrupt microbial metabolism, inhibit growth of bacteria/yeasts/molds, and act as powerful oxygen scavengers preventing oxidation/browning/rancidity.
Where You’ll Find It
E224 (and the broader sulfite family E220-E228) is widely used in beverages and preserved foods:
• Wine (major use; preserves color and flavor; prevents oxidation)
• Cider (major use)
• Beer
• Grape juice
• Fruit juices and juice concentrates
• Dried fruits (apricots, raisins, figs, dates)
• Dried vegetables
• Jams, jellies, and preserves
• Fresh, frozen, and cut vegetables
• Pickles and pickled vegetables
• Lemon juice and citrus juices
• Mushrooms and mushroom products
• Soft drinks and beverages
• Equipment sanitizers for fermenting vessels and wine bottles
Maximum use levels: 10-2000 mg/kg depending on food category. Residual SO₂ strictly regulated.
Note: E224 is preferred over sodium metabisulfite (E223) in some applications because it does not add dietary sodium—an important consideration for those monitoring sodium intake.
🛑 RED SAFETY RATING – SERIOUS HEALTH RISK WARNINGS: E224 has:
• 3-10% of chronic asthmatics experience adverse respiratory reactions (well-established clinical finding)
• 5-10% of asthmatic patients may experience SEVERE or LIFE-THREATENING bronchospasm requiring emergency treatment
• Steroid-dependent asthmatics at significantly elevated risk of severe reactions
• Life-threatening reactions requiring hospitalization and weeks of corticosteroid therapy documented
• Anaphylactic shock documented – potentially fatal allergic-type reactions
• Reactions in 5-10% of asthmatics can be LIFE-THREATENING
• Occupational asthma confirmed: workers handling metabisulfite experience bronchospasm/hospitalization
• TOXICITY RESEARCH FINDING: Produces EXCESSIVE FREE RADICALS; toxic even at lowest recommended ADI concentration
• Causes serious eye damage in direct contact
• Causes skin irritation on contact
• May be harmful if swallowed per safety classification
• EFSA ADI is TEMPORARY and pending re-evaluation due to uncertainties in safety database
• Sulfite sensitivity reactions do NOT respond to antihistamines – avoidance only management
• Sensitivity more common in women and children than men
• Sulfites most common intolerance trigger among chemical food additives
• Mandatory allergen labeling required (one of 14 major allergens in UK)
• Risk of sulfur dioxide formation by reaction with gastric acid after swallowing
E224 has documented serious health risks, particularly for asthmatics. Animal studies show toxicity at the lowest recommended safe level.
Is It Safe?
NO—Not for asthmatics. E224 carries well-documented clinical risks, particularly for asthmatics. While FDA/EFSA/JECFA technically approve it, clinical evidence is clear: 3-10% of asthmatics experience adverse reactions, with 5-10% potentially experiencing life-threatening bronchospasm. Additionally, toxicological research shows E224 produces excessive free radicals and is toxic at the lowest recommended ADI concentration in animal studies. EFSA’s ADI is explicitly temporary, pending re-evaluation. This is NOT a “safe” additive for vulnerable populations.
EFSA’s position (2016 and ongoing):
“The current group ADI of 0.7 mg expressed as SO₂ equivalent/kg bw per day for E220-E228 was TEMPORARY and would be RE-EVALUATED due to UNCERTAINTIES AND LIMITATIONS IN THE DATABASE.”
Additionally, recent animal research found:
“Rats exposed to oral doses for 28 continuous days demonstrated that it is toxic even at the lowest recommended concentration of 0.7 mg/kg body weight. It produces excessive free radicals and has every potential to damage the cells biochemically and structurally when used in slightly greater amounts.”
What Are The Health Concerns?
E224 shares the documented health concerns of the sulfite family, with additional toxicological concerns from recent research:
1. Respiratory Distress in Asthmatics (WELL-DOCUMENTED): 3-10% of chronic asthma patients experience adverse respiratory reactions to sulfites. Symptoms include bronchial constriction, wheezing, chest tightness, coughing, dyspnea (shortness of breath), and respiratory distress.
2. Severe and Life-Threatening Reactions (PROVEN): 5-10% of asthmatic patients may experience severe or even life-threatening bronchospasm requiring emergency treatment, hospitalization, and multiple weeks of corticosteroid therapy. Some cases documented delayed recovery and relapse requiring repeated emergency department visits.
3. High-Risk Asthmatic Subgroups (IDENTIFIED): Steroid-dependent asthmatics and those with marked airway hyperresponsiveness face substantially elevated risk of severe adverse reactions.
4. Anaphylactic Shock (DOCUMENTED): Anaphylactic reactions documented following sulfite exposure—potentially life-threatening.
5. Occupational Asthma (CONFIRMED): Workers handling potassium/sodium metabisulfite experience bronchospasm requiring hospitalization. Occupational asthma well-documented.
6. Excessive Free Radical Production (NEW TOXICOLOGICAL CONCERN): Recent rat studies show E224 produces excessive free radicals, with potential for biochemical and structural cell damage when used in slightly greater amounts than the recommended ADI. This represents a cellular toxicity concern not typical of many food additives.
7. Toxicity at Lowest Recommended ADI (CRITICAL RESEARCH FINDING): Animal studies demonstrate toxicity at the lowest recommended safe concentration (0.7 mg/kg body weight)—a troubling finding suggesting the ADI itself may be set at a level causing cellular damage.
8. Diverse Sensitivity Symptoms (DOCUMENTED): Beyond respiratory symptoms, sulfite sensitivity triggers:
• Dermatitis and skin rash
• Urticaria (hives)
• Flushing
• Hypotension (low blood pressure)
• Abdominal pain and cramping
• Diarrhea and gastrointestinal distress
• Vomiting
• Angioedema (dangerous swelling)
• Nasal congestion
• Sneezing
• Throat symptoms
9. Sex and Age Differences (IDENTIFIED): Sulfite sensitivity may be more common in women and children than men.
10. Asthma-Sulfite Sensitivity Link (WELL-ESTABLISHED): While adverse reactions extremely rare in non-asthmatics, they occur commonly in asthmatics.
11. Chemical Safety Hazards (DIRECT EXPOSURE): E224 causes serious, potentially irreversible eye damage and skin irritation on direct contact.
12. Gastric Reaction Risk: Upon swallowing, E224 reacts with gastric acid to form sulfur dioxide gas—a respiratory irritant that can be absorbed into the lungs.
13. Antihistamine Resistance (TREATMENT IMPLICATION): Sulfite sensitivity reactions do NOT respond to standard antihistamine treatment—avoidance is the only effective management strategy.
14. EFSA Temporary ADI Status (REGULATORY CONCERN): EFSA explicitly states current ADI is temporary, pending re-evaluation due to uncertainties—indicating current safety claims are not definitive.
Comparison to Other Sulfites
E224 (potassium metabisulfite) compared to E223 (sodium metabisulfite):
• Health effects: Identical (both release SO₂ gas; both trigger same respiratory/allergic reactions)
• Main advantage of E224: Does not add dietary sodium (important for those monitoring sodium)
• SO₂ yield difference: E224 yields 57.6% SO₂; E223 yields 67.4% SO₂—making E223 more effective
• Practical use: E224 increasingly preferred in some applications due to sodium concern
• Safety profile: Both part of same sulfite family with identical health risks
Regulatory Status and Inconsistencies
E224 is approved despite documented health concerns and emerging toxicological evidence:
• FDA: GRAS (Generally Recognized as Safe); cannot be used in meats, foods with vitamin B1, or fresh/raw produce
• EFSA: Approved BUT ADI explicitly “temporary” pending re-evaluation
• JECFA: ADI 0-0.7 mg/kg set 1998; approved
• UK/Australia/NZ: Approved
• Mandatory allergen labeling: Sulfites one of only 14 major allergens, requiring prominent label emphasis
• Clinical evidence: 280+ medical publications documenting adverse effects, yet approval continues
• Recent toxicological evidence: Animal studies showing free radical production and toxicity at ADI level, yet approval remains unchanged
Natural Alternatives
Want to avoid E224 and sulfites?
• Sorbic acid (E200) or potassium sorbate (E202) – alternative preservatives without asthma trigger concerns
• Ascorbic acid (E300/Vitamin C) – natural antioxidant
• Rosemary extract – natural antioxidant and preservative
• For wine/cider: Proper pH and temperature management; inert gas (nitrogen/argon) protection
• Refrigeration/Freezing – avoid additives entirely
• Fresh products – accept shorter shelf-life without preservatives
The Bottom Line
E224 (Potassium Metabisulfite) is an approved preservative that carries serious, well-documented clinical health risks, particularly for asthmatics. Clinical evidence confirms 3-10% of chronic asthmatics experience adverse respiratory reactions, with 5-10% potentially experiencing life-threatening bronchospasm requiring hospitalization. Additionally, recent toxicological research shows E224 produces excessive free radicals and is toxic even at the lowest recommended ADI concentration in animal studies—a troubling finding. EFSA’s ADI is explicitly temporary, pending re-evaluation due to uncertainties. E224 is preferred over sodium metabisulfite only because it does not add dietary sodium—not because it is safer. Anaphylactic shock, occupational asthma, and mandatory allergen labeling all reflect serious regulatory concerns. E224 should be strictly avoided by all asthmatics, steroid-dependent patients, and individuals with documented sulfite sensitivity.
Clinical Evidence Strength: 280+ medical publications; 3-10% of asthmatics affected; 5-10% may experience life-threatening reactions.
Documented Respiratory Risk in Asthmatics: Bronchial constriction, wheezing, chest tightness, dyspnea; severe/life-threatening bronchospasm requiring hospitalization documented.
High-Risk Subgroups: Steroid-dependent asthmatics; marked airway hyperresponsiveness; children; women possibly higher risk.
Anaphylaxis Risk: Documented potentially fatal allergic-type reactions.
Occupational Hazard: Workers handling metabisulfite experience bronchospasm and hospitalization; occupational asthma confirmed.
NEW TOXICOLOGICAL CONCERN: Produces excessive free radicals and is toxic at the lowest recommended ADI concentration in animal studies.
EFSA Temporary ADI Status: ADI pending re-evaluation; explicitly temporary due to uncertainties.
Chemical Hazards: Causes serious eye damage and skin irritation; reacts with gastric acid to form SO₂ gas.
Antihistamine Resistance: Reactions do not respond to standard treatments; avoidance only effective strategy.
Mandatory Allergen Labeling: One of 14 major allergens in UK.
Advantage Over Sodium Form: E224 does not add dietary sodium, making it preferable for those monitoring sodium intake—but NOT safer from respiratory/allergic risks.
Recommendation (VERY STRONG): E224 should be strictly avoided by all individuals with asthma, steroid dependency, or documented sulfite sensitivity. The combination of documented clinical harm AND emerging toxicological evidence (free radical production, toxicity at ADI level) makes this additive particularly concerning. EFSA’s explicit statement that the ADI is “temporary” pending re-evaluation is a regulatory red flag. For the general non-asthmatic population, the clinical evidence (280+ publications) and toxicological concerns warrant significant caution. Those concerned about health should prefer alternative preservatives (sorbic acid, ascorbic acid) or properly refrigerated fresh products, particularly when feeding children or managing asthma.