What is E321?
Complete guide to understanding E321 (Butylated Hydroxytoluene) in your food
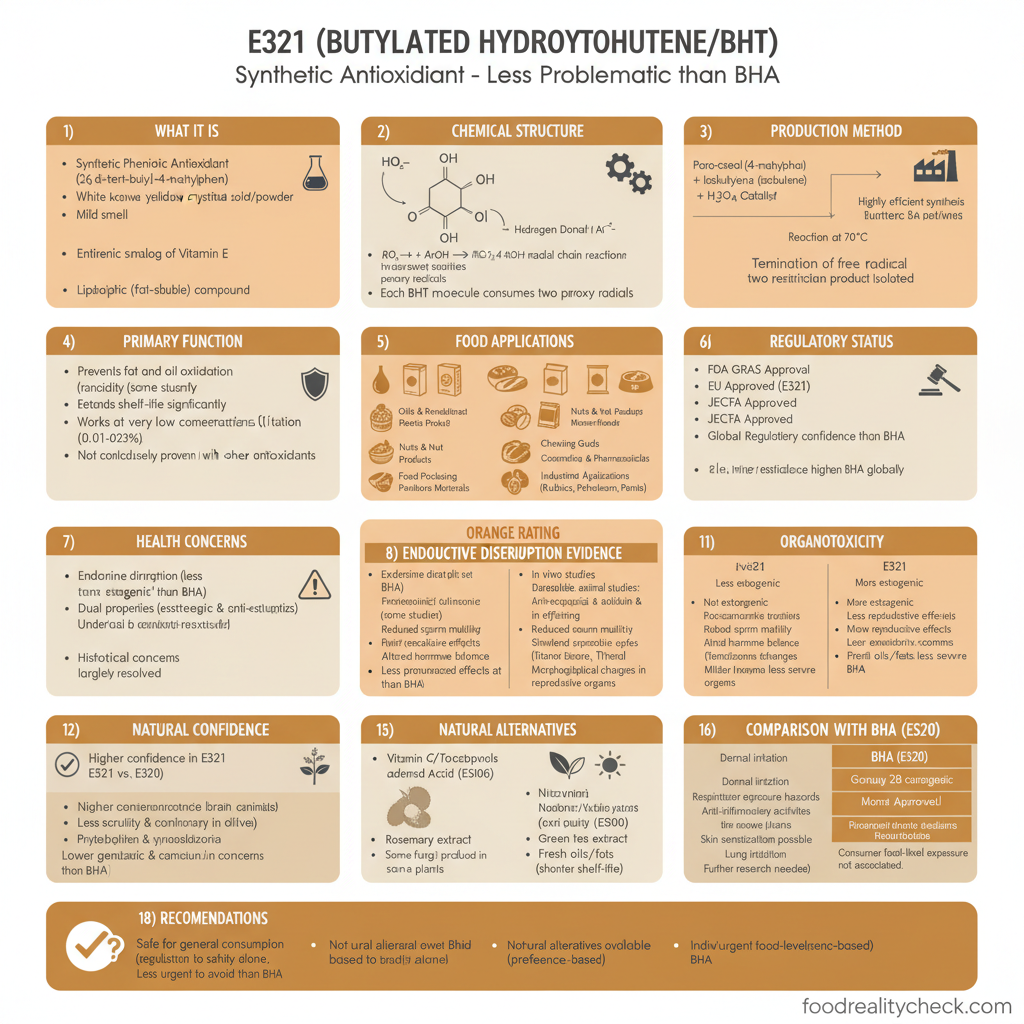
The Quick Answer
E321 is a synthetic antioxidant used to prevent fats and oils from becoming rancid.
It’s used in oils, shortenings, cereals, baked goods, and processed foods.
It is a documented endocrine disruptor with reproductive and developmental toxicity concerns, contradictory carcinogenicity evidence, and significant toxicological uncertainty—making it one of the most controversial approved food additives, with regulatory agencies recommending caution and some suggesting avoidance.
📌 Quick Facts
- Category: Synthetic phenolic antioxidant preservative
- Full Name: Butylated Hydroxytoluene (2,6-di-tert-butyl-4-methylphenol)
- Chemical Formula: C₁₅H₂₄O
- Source: Entirely synthetic, produced from phenol and isobutylene
- Found in: Oils, shortenings, cereals, baked goods, nuts, snacks, instant soups, processed foods, chewing gum
- Safety Status: FDA GRAS approval; EU approved but controversial; EFSA ADI 0.25 mg/kg (raised from lower levels despite concerns)
- Critical Concerns: Endocrine disruption, reproductive/developmental toxicity, carcinogenicity uncertainty, organ damage
- CSPI Rating: “Caution” – Recommends avoiding due to safety uncertainty
What Exactly Is It?
E321 is a synthetic phenolic compound (BHT) chemically produced from phenol and isobutylene.
Its full chemical name is 2,6-di-tert-butyl-4-methylphenol.
It appears as a white crystalline solid with a mild phenolic, camphor-like odor.
E321 works by inhibiting free radical autoxidation—the chemical process that causes fats and oils to become rancid. It donates hydrogen atoms to break free radical chain reactions.
E321 is entirely synthetically manufactured—there is no natural form, though trace amounts occur naturally in some plants (Thymus longicaulis, Teucrium leucocladum).
E321 is chemically related to BHA (E320) and like BHA, is a substituted phenolic compound structurally similar to disinfectants and wood preservatives.
Where You’ll Find It
E321 appears in many preserved and processed foods:
• Vegetable oils and animal fats
• Shortenings and margarines
• Cereals and breakfast products
• Baked goods (cakes, cookies, pastries)
• Nuts and snack foods
• Potato chips and crisps
• Instant soups and broths
• Processed meats
• Dried potatoes
• Chewing gum
• Powdered milk
• Seasonings and spice mixes
• Cosmetics and pharmaceuticals
• Industrial applications (biodiesel, plastics)
E321 is often used in combination with BHA (E320) and propyl gallate (E310) for synergistic preservation effects.
Why Do Food Companies Use It?
E321 serves critical preservation functions:
Prevents fat rancidity: Fats and oils naturally oxidize when exposed to air, producing rancid off-flavors and potentially toxic oxidation products. E321 prevents this oxidation, extending shelf-life by months or years.
Highly effective: Works at very low concentrations (0.01–0.02% for animal fats).
Cost-effective: Cheaper than most natural antioxidants.
Heat stable: Resists degradation at high temperatures used in food processing and cooking.
Synergistic with other additives: Works well in combination with BHA (E320) and propyl gallate (E310).
Is It Safe?
No—E321 is officially approved but highly controversial due to documented endocrine disruption, reproductive and developmental toxicity, contradictory carcinogenicity evidence, and significant regulatory uncertainty. Multiple health concerns warrant caution.
The FDA classifies E321 as GRAS (Generally Recognized as Safe).
The EFSA approved E321 with an ADI of 0.25 mg/kg body weight per day (this ADI was raised from lower levels in 2012 despite ongoing safety concerns).
However, the Center for Science in the Public Interest (CSPI) rates E321 as “Caution” and recommends avoiding it.
⚠️ CRITICAL HEALTH CONCERNS – Why E321 Is Controversial:
1. ENDOCRINE DISRUPTION: E321 acts as an endocrine disruptor, interfering with hormone signaling. Studies show disruption of estrogen and androgen pathways, similar to E320 (BHA).
2. REPRODUCTIVE & DEVELOPMENTAL TOXICITY: Animal studies show:
• Reproductive organ damage at moderate doses
• Effects on sperm quality and fertility
• Impacts on sexual maturation
• Developmental effects in offspring
A small fraction of the general population may exceed the ADI and face reproductive health risks
3. CARCINOGENICITY UNCERTAINTY: Evidence is contradictory and confusing:
• 1979 National Cancer Institute study: No cancer risk in mice
• WHO (1986): Discussed possible link to cancer
• Long-term animal studies: Showed both cancer-promoting AND cancer-inhibiting effects depending on dose, organ, and animal type
• EFSA (2012): Concluded “not of concern” for carcinogenicity, but acknowledged tumor promotion effects in some studies
• This contradiction led CSPI to recommend caution despite regulatory approval
4. ORGAN TOXICITY: Studies show:
• Liver damage and increased liver weight
• Kidney damage at high doses
• Lung toxicity (in topical application studies)
• Decreased enzyme activity in liver at lower doses
5. METHEMOGLOBINEMIA RISK (ACUTE): Both BHA (E320) and BHT (E321) can cause life-threatening cyanosis (methemoglobinemia) at high doses—oxygen binding in red blood cells is prevented, causing dangerous oxygen deficiency. This is particularly concerning in children.
6. IMMUNE SYSTEM EFFECTS: Studies show BHT enhances and inhibits immune response depending on conditions—creating uncertainty about long-term immune impacts.
What Are The Health Concerns?
E321 has multiple documented and serious health concerns:
Endocrine disruption: Acts as endocrine disruptor interfering with hormone signaling pathways.
Reproductive toxicity: Animal studies show reproductive organ damage, reduced fertility, impaired sperm quality, effects on sexual maturation at moderate doses.
Developmental toxicity: Fetal exposure causes developmental effects; offspring show altered maturation.
Carcinogenicity (contradictory evidence): Evidence is conflicting—some studies show promotion of tumors, others show inhibition or no effect. This contradiction prevents clear regulatory assessment. EFSA concluded “not of concern” but acknowledged complexity.
Organ toxicity: Liver damage (increased weight, enzyme changes), kidney damage at high doses, lung toxicity in topical exposure studies.
Methemoglobinemia (acute, high-dose): Can cause dangerous oxygen-binding impairment in red blood cells, particularly in children.
Immune system effects (uncertain): Studies show contradictory effects on immune function, creating uncertainty about long-term impacts.
Vitamin K destruction: Can destroy Vitamin K, affecting blood clotting.
Migraines: Reported association with migraine attacks in some individuals.
Allergic reactions: Can cause allergic reactions in sensitive individuals.
Natural vs Synthetic Version
E321 is entirely synthetic—there is no natural form.
It’s manufactured from phenol and isobutylene through chemical synthesis. While trace amounts occur naturally in some plants, all food-grade E321 is synthetically produced.
Natural Alternatives
Want to avoid E321?
Natural antioxidants include:
• Vitamin E/Tocopherols (E306) – natural antioxidant
• Vitamin C/Ascorbic Acid (E300) – natural antioxidant
• Rosemary extract – natural antioxidant
• Green tea extract – natural antioxidant
• Oregano extract – natural antioxidant
• Citric acid (E330) – synergistic with antioxidants
• Fresh oils with shorter shelf-life – accept reduced shelf-life
Natural alternatives are less potent and more expensive but avoid the documented reproductive, endocrine, and carcinogenicity concerns of E321.
The Bottom Line
E321 (Butylated Hydroxytoluene) is a synthetic antioxidant that is officially approved but highly controversial due to documented endocrine disruption, reproductive and developmental toxicity, contradictory carcinogenicity evidence, and significant regulatory uncertainty—with the Center for Science in the Public Interest recommending avoidance.
Regulatory Red Flag: EFSA RAISED the ADI from previous lower levels in 2012 despite ongoing safety concerns and contradictory carcinogenicity evidence—suggesting regulatory compromise rather than genuine safety confidence.
Contradictory Evidence: The mixture of cancer-promoting and cancer-inhibiting findings in animal studies, combined with reproductive/developmental toxicity, creates genuine scientific uncertainty unsuitable for casual approval.
Endocrine Disruption Confirmed: E321 is a documented endocrine disruptor—a class of chemicals increasingly recognized as particularly hazardous due to impacts on hormone systems at low doses.
Reproductive Concerns: Animal studies confirm impacts on fertility, sperm quality, and reproductive organ development—concerns regulatory agencies have not adequately emphasized.
CSPI Assessment: The Center for Science in the Public Interest rates E321 as “Caution” and recommends avoiding it—reflecting expert doubt about safety despite regulatory approval.
If You Want to Minimize Exposure: Avoid E321-containing foods (check labels for “BHT” or “E321”), particularly processed oils, cereals, baked goods, and nuts. Choose fresh products or natural antioxidant-preserved alternatives.
For Pregnant Women & Children Particularly: Given documented reproductive and developmental toxicity, avoiding E321 during pregnancy and in children’s diets is prudent.
Future Outlook: E321 faces increasing regulatory scrutiny. The EU may impose tighter restrictions or removal from approval as endocrine disruptor concerns intensify. FDA may eventually revisit its GRAS classification given emerging evidence.