What is E404?
Complete guide to understanding E404 (Calcium Alginate) in your food
The Quick Answer
E404 is calcium alginate, a natural thickener, stabilizer, gelling agent, and emulsifier derived from brown seaweed.
It’s used in food to create smooth textures, prevent ingredients from separating, form heat-stable gels, and prevent ice crystal formation in frozen products.
Most people consume it multiple times per week in ice cream, frozen desserts, jams, sauces, and other processed foods.
📌 Quick Facts
- Category: Thickener, Stabilizer, Emulsifier, Gelling Agent, Anti-caking Agent
- Source: Brown seaweed (Phaeophyta) harvested globally
- Found in: Ice cream, frozen desserts, jams, sauces, meat products, dairy products, beverages
- Safety: Approved safe by FDA, EFSA, JECFA with no specific ADI set
- Natural or Synthetic: Natural—extracted directly from seaweed
- Key Advantage: Forms heat-stable, water-insoluble gels; prevents ice crystallization in frozen foods
What Exactly Is It?
E404 is calcium alginate, the calcium salt of alginic acid, a naturally occurring polysaccharide found in brown seaweed.
Calcium alginate has the chemical formula (C₆H₇O₆Ca)ₙ. It’s extracted from brown seaweed species including Macrocystis pyrifera (giant kelp), Laminaria digitata, Laminaria cloustoni, and Ascophyllum nodosum. The alginic acid is extracted from the seaweed cell walls and then treated with calcium compounds (most commonly calcium chloride or calcium carbonate) to create calcium alginate.
Unlike its soluble cousins (sodium, potassium, and ammonium alginates), calcium alginate is water-insoluble, appearing as a white to cream-colored powder or granules. This insolubility is actually its strength—when calcium alginate contacts solutions containing calcium ions or acid, it forms strong, heat-stable gels with a gelatinous texture.
In technical terms, calcium alginate is a linear copolymer of α-L-guluronic acid (G units) and β-D-mannuronic acid (M units). The unique property of calcium alginate is its ability to form immediately into gels through a process called “ionic gelation” when it encounters calcium ions, making it ideal for creating structured foods like jellies and for preventing unwanted crystallization in frozen products.
Where You’ll Find It
E404 appears in a wide range of processed foods, particularly those requiring freeze protection and gel formation:
• Ice cream and frozen desserts (primary use—prevents ice crystal formation)
• Frozen bakery products and cakes
• Jams, jellies, marmalades, and fruit preserves
• Candy and confectionery with gel centers
• Yogurt and dairy products
• Sauces, gravies, and condiments
• Mayonnaise and salad dressings
• Aspics and meat jellies (meat products)
• Pâtés and processed meat products
• Soups and vegetable preparations
• Beer and wine (clarification and stabilization)
• Soft drinks and fruit juices
• Puddings and desserts
• Cereal-based baby foods (in limited quantities)
• Processed fruits and vegetables
• Innovative culinary applications (vegetable caviar, fruit balls)
If you eat ice cream, frozen desserts, jam, or processed foods regularly, you’ve likely consumed E404 multiple times this week.
Why Do Food Companies Use It?
E404 performs six critical functions in food:
1. Ice crystal prevention in frozen products: This is E404’s signature function. In ice cream and frozen desserts, it prevents the formation of large ice crystals that create a grainy, unpleasant texture during storage and freezing cycles. It keeps frozen products smooth and creamy for months.
2. Heat-stable gel formation: Unlike many thickeners that break down at high temperatures, calcium alginate forms strong gels that remain stable even when heated. This is invaluable in products like aspics and jellies that may be heated during processing or served warm.
3. Acid stability: E404 maintains its thickening and gelling properties in acidic environments, making it ideal for jams (naturally acidic due to fruit) and acidic beverages where other thickeners might fail.
4. Emulsion stabilization: It prevents oil and water from separating in emulsified products like mayonnaise, salad dressings, and sauces, maintaining a uniform appearance and preventing visual separation during storage.
5. Water-binding and moisture retention: It binds water molecules, maintaining moisture content in products and preventing them from drying out during storage, which extends shelf life.
6. Texture creation for innovative foods: Due to its strong gel-forming properties, E404 is used in cutting-edge culinary applications like vegetable caviar (tiny gel spheres) and fruit balls, where precise texture control is essential.
Without E404, ice cream would develop an icy, grainy texture within weeks of storage. Jams would separate, sauces would thin out, and many processed foods would deteriorate in quality.
Is It Safe?
E404 is considered very safe by all major regulatory authorities.
The FDA, EFSA, and the Joint FAO/WHO Expert Committee on Food Additives (JECFA) have all approved calcium alginate for food use. No specific Acceptable Daily Intake (ADI) limit has been established—meaning regulatory agencies found no safety concern even at very high consumption levels.
JECFA recognizes alginates (including calcium alginate) as safe at consumption levels of up to 50 mg/kg of body weight per day. EFSA’s 2017 safety re-evaluation concluded “no safety concern and no need to establish a numerical ADI,” reflecting the additive’s excellent safety profile.
Special considerations: At very high intake levels, calcium alginate may cause a mild laxative effect (like other polysaccharide bulking agents) and could reduce absorption of calcium, iron, and zinc. However, these effects occur only when consumption greatly exceeds approved food-use levels. In practical food consumption, this is not a concern.
Infant and young children’s foods: E404 is permitted in baby foods, but only in limited amounts and specifically in puddings and desserts based on processed cereals. This restricted use reflects abundance of caution in vulnerable populations, though no specific safety concerns have been identified at these low levels.
E404 vs Other Alginates: Key Differences
The alginate family (E401-E404) all come from brown seaweed but have different properties based on their mineral base:
E401 (Sodium alginate): Soluble in water. General-purpose thickener and stabilizer. Most widely used.
E402 (Potassium alginate): Soluble in water. Used in low-sodium products. Similar to E401 but tailored for sodium reduction.
E403 (Ammonium alginate): Soluble in water. Heat and acid resistant. Restricted from infant foods.
E404 (Calcium alginate): Water-insoluble. Forms heat-stable gels. Prevents ice crystallization. Primarily for frozen and gelled products.
E404’s defining feature is its water-insolubility and ability to form gels—making it uniquely suited for ice cream, frozen desserts, and structured gel products.
Natural vs Synthetic Version
E404 is entirely natural—there is no synthetic version.
Calcium alginate is extracted directly from brown seaweed through a physical and chemical process: seaweed is washed with water and acid to extract the alginic acid, then treated with calcium chloride or calcium carbonate to convert it to calcium alginate. The insoluble gel is then filtered, washed, dried, and ground into a powder.
There is no laboratory-created or synthetic alternative—E404 is harvested and refined from nature, making it one of the few food additives that is genuinely 100% natural in origin.
Historical and Innovative Uses
Beyond traditional food applications, calcium alginate has expanded uses:
Medical applications: Calcium alginate is widely used in wound dressings because its gels have hemostatic (blood-stopping) properties and accelerate wound healing. It’s one of the most common wound dressing materials in hospitals.
Pharmaceutical tablets and capsules: Used to control the release rate of active ingredients in medications and dietary supplements.
Cosmetic formulations: Used in creams, masks, and lotions. Professional alginate face masks are popular in spas and salons for their skin-tightening properties.
Innovative food technology: Spherification (creating tiny gel spheres) using calcium alginate is a molecular gastronomy technique used in high-end restaurants and food science to create unique textures and presentations.
Environmental and Sustainability
Brown seaweed is a renewable resource that grows rapidly in coastal waters worldwide. Harvesting for alginate production doesn’t deplete fish stocks or significantly harm marine ecosystems—unlike some fishing practices. The seaweed regenerates quickly, making production sustainable. Major producers include China, Japan, France, the USA, UK, Chile, and Norway.
Natural Alternatives
Want to avoid E404? Food companies sometimes use these alternatives:
• E401 (Sodium alginate) or E402 (Potassium alginate): Soluble forms with similar properties, though they don’t form heat-stable gels as effectively
• Xanthan gum (E415): A natural polysaccharide from fermentation, different thickening properties
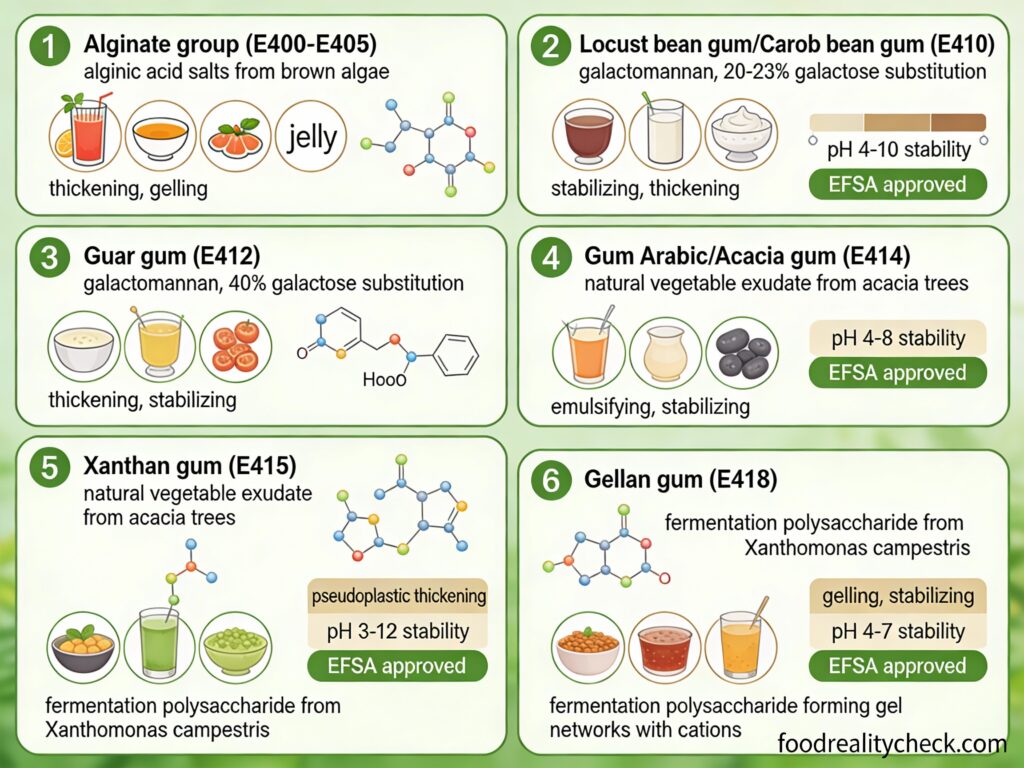
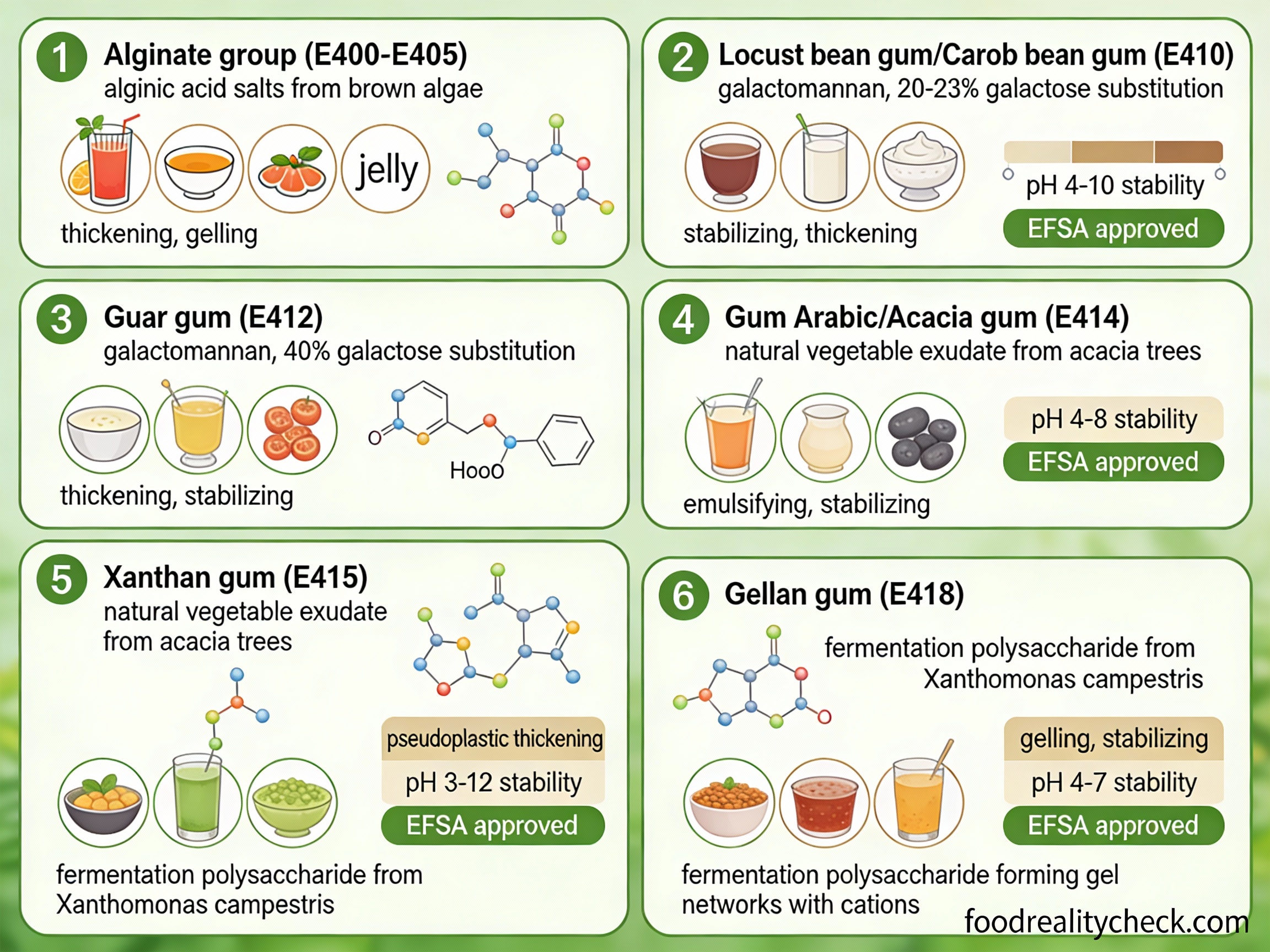
• Locust bean gum (E410): Extracted from carob seeds
• Guar gum (E412): From guar bean seeds
• Gelatin: Animal-based gelling agent (not suitable for vegans/vegetarians)
• Pectin: Plant-based gelling agent from fruit
• Agar: Seaweed-based gelling agent
• Carrageenan (E407): Another seaweed-derived gelling agent
These alternatives have similar thickening or gelling functions but different heat stability and gel characteristics. For ice cream and frozen applications specifically, E404’s ice crystal prevention capability is difficult to replicate with other additives.
The Bottom Line
E404 (calcium alginate) is a safe, natural food additive derived directly from brown seaweed.
Its defining feature is the formation of heat-stable, water-insoluble gels and its exceptional ability to prevent ice crystal formation in frozen products. It has been approved by all major regulatory agencies worldwide and has an excellent safety profile with no identified health risks at food-use levels. Unlike many food additives, no ADI limit was deemed necessary—reflecting regulatory confidence in its safety.
Calcium alginate appears primarily in ice cream, frozen desserts, jams, sauces, and meat products where its unique gel-forming and texture-stabilizing properties are essential. It’s suitable for vegans and vegetarians, has no known religious dietary restrictions, and has demonstrated potential health benefits including cholesterol reduction and heavy metal elimination.
E404 is one of the safest and most thoroughly studied food additives available. For the general population, including children, E404 poses no identified health risk when consumed in normal food amounts. If you’re looking to minimize additives, reducing processed food intake would lower exposure, but the additive itself poses no health concerns.