What is E966?
Complete guide to understanding E966 (Lactitol) in your food
The Quick Answer
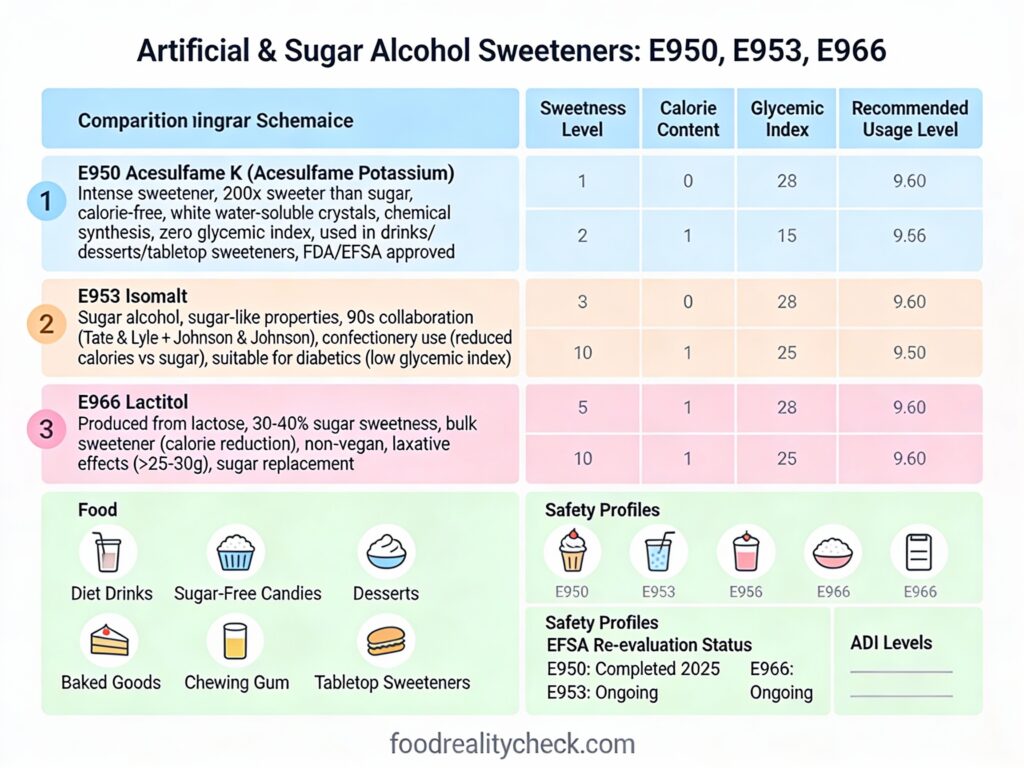
E966 is lactitol, a sugar alcohol (polyol) derived from lactose that provides sweetness at only 30-40% of sugar’s intensity, while contributing minimal calories and having laxative properties.
It’s used as a bulk sweetener in sugar-free and reduced-calorie foods—particularly valuable in baked goods, confectionery, and ice cream where its stability and non-hygroscopic properties are advantageous.
Most people consuming sugar-free products occasionally encounter E966, though it’s less common than some other polyols due to its low sweetness requiring combination with intense sweeteners for palatable taste.
📌 Quick Facts
- Category: Sugar Alcohol (Polyol), Bulk Sweetener, Laxative (medical use), Prebiotic
- Source: Synthetically produced from lactose (milk sugar) through hydrogenation
- Found in: Sugar-free candies, baked goods, chocolate, ice cream, chewing gum, beverages, dietary/medical products
- Safety: FDA GRAS approved; EFSA approved; JECFA ADI “not specified” (safe at any practical level)
- Natural or Synthetic: Semi-synthetic (derived from natural lactose via hydrogenation)
- Sweetness Level: 30-40% as sweet as sugar (lowest among polyol sweeteners)
- Calories: 2-2.5 calories per gram (vs. 4 for sugar); 40-60% caloric reduction
- Medical Use: FDA-approved osmotic laxative for chronic constipation (Importal brand)
What Exactly Is It?
E966 is lactitol, chemically known as 4-O-β-D-galactopyranosyl-D-glucitol—a disaccharide sugar alcohol produced from lactose through selective reduction of the glucose moiety.
Lactitol is composed of a galactose unit (from lactose) bound to sorbitol (hydrogenated glucose), creating the molecule C₁₂H₂₄O₁₁ with molecular weight of 344.3 g/mol. This structure gives lactitol a sweetness of only 30-40% compared to sucrose, significantly lower than other polyols like sorbitol (50-60%), maltitol (75-90%), or xylitol (100%).
Physically, lactitol appears as a white crystalline powder or colorless liquid (in solution form, typically 70% concentration). It is readily soluble in water but only slightly soluble in ethanol. Unlike many other polyols, lactitol is nonhygroscopic (does not absorb moisture from air), making it particularly valuable for maintaining texture stability in stored confectionery and baked goods.
Lactitol has a melting point of 162-164°C, making it heat-stable for baking and cooking applications. The compound is remarkably stable—resistant to degradation by heat, light, acid, and alkaline conditions, making it suitable for a wide range of food processing methods.
The most distinctive property of lactitol is its incomplete absorption in the small intestine due to the lack of suitable lactase enzymes to hydrolyze the disaccharide bond. This results in most lactitol reaching the colon intact, where it is extensively fermented by colonic microbiota, creating both beneficial and potential side effects.
Where You’ll Find It
E966 appears in select sugar-free and reduced-calorie products:
• Sugar-free candies and hard-boiled sweets
• Sugar-free chocolate and chocolate coatings
• Sugar-free baked goods (cakes, cookies, bread)
• Sugar-free chewing gum
• Sugar-free ice cream and frozen desserts
• Sugar-free confectionery and sweets
• Dietetic foods for diabetics
• Weight-control foods
• Sugar-free beverages
• Biscuits and crackers (sugar-free varieties)
• Medical/pharmaceutical products (Importal and other laxatives)
• Nutritional supplements
• Oral dosage forms (excipient in tablets)
• Cosmetic products
• Food supplements and functional foods
E966 is less commonly encountered than isomalt (E953) or maltitol (E965) in food applications, partly because its low sweetness (30-40% of sugar) requires combination with other sweeteners for acceptable taste.
Why Do Food Companies Use It?
E966 performs four critical functions, particularly in specialized applications:
1. Bulk sweetening with stability: Unlike intense sweeteners (aspartame, sucralose) which provide only taste, lactitol provides bulk—allowing manufacturers to maintain texture, mouthfeel, and product structure similar to sugar-sweetened products. Combined with intense sweeteners, lactitol (70%) + aspartame (0.03%) creates sugar-like taste and texture.
2. Heat and storage stability: Lactitol’s exceptional stability under heat, light, acid, and alkaline conditions makes it ideal for baked goods and products requiring extended shelf life. Its nonhygroscopic nature prevents moisture absorption that would create stickiness or texture degradation.
3. Prebiotic and health benefits: Unlike most sweeteners which provide only taste, lactitol’s colonic fermentation produces beneficial short-chain fatty acids (acetate, propionate, butyrate) that support colonic health and beneficial microbiota—providing genuine health benefits similar to dietary fiber.
4. Milk product origin appeal: Derived from lactose (milk sugar), lactitol appeals to manufacturers seeking “milk-derived” or “natural” sweetener origins for marketing purposes.
Why it’s used selectively: The very low sweetness (30-40% of sugar) requires combination with other sweeteners, increasing formulation complexity. For simple sweetening needs, higher-sweetness polyols (maltitol at 75-90%, isomalt at 50-60%) or intense sweeteners are often preferred.
Is It Safe?
E966 is considered very safe with favorable regulatory approval and minimal toxicological concerns.
Regulatory Status:
• FDA (USA): Recognized as GRAS (Generally Recognized As Safe); approved as a direct food additive; also approved as an osmotic laxative medication (Importal, February 2020)
• EFSA (Europe): Approved as direct food additive (E966)
• JECFA (WHO/FAO): ADI “not specified”—indicating safety at any practical consumption level
⚠️ Laxative Effect—Primary Safety Consideration: Lactitol’s most significant characteristic is its strong laxative action at moderate intakes. Unlike isomalt (which causes GI effects above 20-30 g) or sorbitol (which causes effects above 10-15 g), lactitol is therapeutically used as a laxative at 10-20 g daily doses.Documented gastrointestinal effects include:
• Osmotic diarrhea: Primary mechanism—unabsorbed lactitol draws water into the colon, promoting bowel movements
• Threshold dose: Significant GI effects may occur above 5-10 g daily in sensitive individuals; become pronounced above 15-20 g
• Bloating and gas: Colonic fermentation produces gases (H₂, CO₂, methane) causing abdominal distension
• Abdominal pain and cramping: More pronounced than with other polyols at equivalent doses
• Individual variation: Effects depend on individual gut microbiota and adaptation—regular consumers develop increased tolerance
• Medical approval: Precisely because of these laxative properties, lactitol was approved by FDA (February 2020) as an osmotic laxative for treating chronic idiopathic constipation (CIC)
EU labeling requirement: Products containing lactitol must carry warning “Excessive consumption may produce laxative effects.”
Other documented considerations:
• Glycemic impact: Negligible—minimal blood glucose rise (no insulin response)
• Dental health: Non-cariogenic; promotes dental health (much less cariogenic than sucrose)
• Caloric contribution: 2-2.5 calories per gram (40-60% of sugar’s 4 calories per gram)
• Prebiotic effects: Fermentation-produced short-chain fatty acids support colonic health
• Milk allergy consideration: Derived from lactose (milk), though lactose is largely absent after hydrogenation; individuals with severe milk allergies should verify manufacturing process
Unique Property: Laxative Agent and Medical Use
E966 lactitol is unique among food sweeteners in having FDA-approved medical status as an osmotic laxative medication.
In February 2020, the FDA approved lactitol monohydrate as an osmotic laxative for treating chronic idiopathic constipation (CIC) in adults—a classification that recognizes its therapeutic laxative properties. Marketed under brand names including Importal and others, lactitol at therapeutic doses (10-20 g daily) is prescribed as a medical treatment, not merely a food additive.
This dual status (food sweetener at low intakes; laxative medication at therapeutic intakes) creates an unusual situation where the same compound is both a food ingredient and a pharmaceutical agent depending on dose.
Production Process
E966 lactitol is produced through catalytic hydrogenation of lactose:
1. Lactose (milk sugar) is dissolved in water
2. The solution is hydrogenated under pressure and catalysis using Raney nickel catalyst—this reduces the glucose component of lactose to glucose alcohol (sorbitol) while preserving the galactose unit
3. The resulting product is a mixture of disaccharide sugar alcohols with the predominant structure being galactose-sorbitol (lactitol)
4. The solution is purified to remove unreacted lactose and byproducts
5. The purified solution is crystallized or dried to produce either crystalline lactitol monohydrate or lactitol solution (70% concentration)
6. The final product is standardized and packaged
The process is a selective chemical reduction without introduction of foreign reagents—using only hydrogen gas and a catalyst.
Natural vs Synthetic Version
E966 is semi-synthetic—derived from natural lactose but requiring chemical transformation.
Like isomalt, lactitol occupies a middle ground between natural and synthetic: it’s made from naturally-derived lactose (milk sugar) but requires hydrogenation to produce. Lactitol does not exist in nature as an isolated product—it must be manufactured. This semi-synthetic status makes it neither purely natural nor fully synthetic.
Comparison with Other Polyols
Lactitol’s very low sweetness distinguishes it from other sugar alcohols:
• Sorbitol (E420): 50-60% sweetness; 2.6 calories/g; hygroscopic; laxative threshold ~10-15 g
• Mannitol (E421): 50-70% sweetness; 1.6 calories/g; hygroscopic; cooling sensation
• Isomalt (E953): 50-60% sweetness; 2 calories/g; low hygroscopicity; laxative threshold ~20-30 g
• Maltitol (E965): 75-90% sweetness; 2.1-2.7 calories/g; hygroscopic; laxative threshold ~15-20 g
• Xylitol (E967): 100% sweetness; 2.4 calories/g; hygroscopic; cooling sensation; expensive
• Erythritol (E968): 70% sweetness; 0.2 calories/g; no laxative effect; cooling sensation
Lactitol’s position at the lowest sweetness level (30-40%) makes it unique among polyols—it cannot substitute for sugar in simple sweetening applications but excels in specific formulations where its other properties (stability, bulk, laxative action) are advantageous.
Medical Applications Beyond Food
E966 lactitol has substantial medical applications:
• Osmotic laxative: FDA-approved for chronic idiopathic constipation treatment (Importal brand)
• Hepatic encephalopathy treatment: Used clinically to lower ammonia levels in patients with cirrhosis
• Pharmaceutical excipient: Listed excipient in some prescription medications
• Oral dosage form: Direct-compression form used in tablet manufacturing
• Combination therapy: Combined with Ispaghula husk (psyllium) for constipation treatment
This medical dual-use is uncommon among food additives, reflecting lactitol’s distinctive biological activity.
Prebiotic Benefits and Colonic Health
Unlike most sweeteners providing only taste, lactitol offers documented colonic health benefits:
• Short-chain fatty acid production: Colonic fermentation produces butyrate, propionate, and acetate—beneficial metabolites supporting colonic health
• pH reduction: Fermentation reduces colonic pH, creating environment favoring beneficial bacteria
• Microbiota composition improvement: Promotes growth of beneficial Bifidobacterium and Lactobacillus species
• Pathogen reduction: Acidic colonic environment inhibits growth of potential pathogens
• Prebiotic classification: Functions similarly to other prebiotic fibers in supporting beneficial microbiota
These prebiotic properties differentiate lactitol from non-fermentable sweeteners, providing genuine health benefits comparable to dietary fiber consumption.
Environmental and Sustainability
Lactitol production uses lactose (from milk processing, a byproduct) as raw material, representing a circular economy application of a waste stream. Hydrogenation requires hydrogen gas and catalysts, creating moderate environmental footprint. Two main manufacturers (Danisco and Purac Biochem) produce approximately 10,000 tons annually, meeting most global demand. Overall sustainability is moderate—acceptable but not exceptional compared to directly extracted additives.
Natural Alternatives
Want to avoid E966? Food companies sometimes use these alternatives:
• E953 (Isomalt): Higher sweetness (50-60%); lower laxative threshold
• E965 (Maltitol): Much higher sweetness (75-90%); more commonly used
• E967 (Xylitol): Higher sweetness (100%); no laxative effect; more expensive
• E968 (Erythritol): Similar sweetness; zero calories; no laxative effect
• E420 (Sorbitol): Similar sweetness; lower cost; more hygroscopic
• E960 (Stevia): Natural intense sweetener; lacks bulk
• E955 (Sucralose): Artificial sweetener; lacks bulk
• Sugar itself: Full-calorie traditional sweetener
For formulations requiring lactose-derived sweetener or those seeking laxative properties, lactitol remains unique. For simple bulk sweetening, maltitol or isomalt are more commonly used due to higher sweetness.
The Bottom Line
E966 (lactitol) is a semi-synthetic sugar alcohol derived from lactose that is FDA GRAS approved, FDA-approved as an osmotic laxative, EFSA approved, and JECFA ADI “not specified,” providing minimal sweetness (30-40% of sugar) while offering significant laxative and prebiotic properties.
Lactitol functions as a bulk sweetener in sugar-free and reduced-calorie foods, particularly valued in baked goods and confectionery for its exceptional stability, non-hygroscopic properties, and compatibility with other sweeteners. It provides 2-2.5 calories per gram (40-60% caloric reduction versus sugar) with negligible glycemic impact and no insulin response—suitable for diabetics.
The primary characteristic distinguishing lactitol from other polyols is its strong laxative action at moderate intakes (5-10 g and above)—precisely the property that led to FDA approval as an osmotic laxative medication (February 2020) for chronic constipation treatment. This dual status (food additive and laxative medication) is unique among sweeteners.
Beyond its functional role, lactitol offers genuine health benefits as a prebiotic, promoting colonic fermentation that produces beneficial short-chain fatty acids and supports beneficial gut microbiota composition. These prebiotic effects are similar to dietary fiber consumption.
The very low sweetness (30-40% of sugar) requires combination with intense sweeteners in most applications, limiting its use primarily to formulations where its other properties (stability, bulk, laxative action, prebiotic effects) justify the added complexity. For consumers seeking sugar-free alternatives with meaningful health benefits beyond simple sweetness, lactitol offers distinctive advantages, though its strong laxative properties warrant attention to intake levels.